Summary
The kinetics and mechanism of the system: [FeL(OH)]2−n + 5 CN− ⇌ [Fe(CN)5(OH)]3− + Ln−, where L=DTPA or HEDTA, have been investigated at pH= 10.5±0.2, I=0.25 M and t=25±0.1 ‡C.
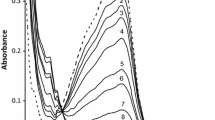
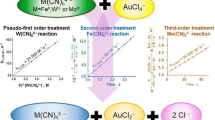
As in the reaction of [FeEDTA(OH)]2−, the formation of [Fe(CN)5(OH)]3− through the formation of mixed ligand complex intermediates of the type [FeL(OH)(CN)x]2−n−x, is proposed. The reactions were found to consist of three observable stages. The first involves the formation of [Fe(CN)5(OH)]3−, the second is the conversion of [Fe(CN)5(OH)]3− into [Fe(CN)6]3− and the third is the reduction of [Fe(CN)6]3− to [Fe(CN)6]4− by oxidation of Ln− The first reaction exhibits a variable order dependence on the concentration of cyanide, ranging from one at high cyanide concentration to three at low concentration. The transition between [FeL(OH)]2−n and [Fe(CN)5(OH)]3− is kinetically controlled by the presence of four cyanide ions around the central iron atom in the rate determining step. The second reaction shows first order dependence on the concentration of [Fe(CN)5(OH)]3− as well as on cyanide, while the third reaction follows overall second order kinetics; first order each in [Fe(CN)6]3− and Ln−, released in the reaction. The reaction rate is highly dependent on hydroxide ion concentration.
The reverse reaction between [Fe(CN)5(OH)]3− and Ln− showed an inverse first order dependence on cyanide concentration along with first order dependence each on [Fe(CN)5− (OH)]3− and Ln−. A five step mechanism is proposed for the first stage of the above two systems.
Similar content being viewed by others
References
D. W. Margerum, T. J. Bydalek and J. J. Bishop,J. Am. Chem. Soc., 83, 1751 (1961).
S. Nakamura,Ph. D. Thesis, Univ. of Chicago, 1964.
J. P. Jones and D. W. Margerum,Inorg. Chem., 8, 1486 (1969).
R. E. Hamm and J. P. Tompleton,Inorg. Chem., 12, 755 (1973).
D. W. Margerum and L. P. Morgenthalar,J. Am. Chem. Soc., 84, 706 (1962).
J. Burgess,Inorg. Chim. Acta, 5, 133 (1971).
J. Burgess,J. Chem. Soc., Dalton Trans., 1061 (1972).
H. C. Bajaj and P. C. Nigam,Transition Met. Chem., 7, 190 (1982).
H. C. Bajaj and P. C. Nigam,Transition Met. Chem., 8, 109 (1983).
G. Brauer,Handbook of Preparatory Inorganic Chemistry, 2nd Edit., Academic Press, N. Y. 1965, p. 1511.
G. Davies and A. R. Garafalo,Inorg. Chem., 15, 1103 (1976).
G. Schwarzenbach,Complexometric Titrations, Int. Sci. Pub., N. Y., 1955, p. 77.
A. I. Vogel,Textbook of Quantitative Analysis, 3rd Edit., Longman Green, London, 1962, p. 270.
B. Jaselskis,J. Am. Chem. Soc., 83, 1082 (1961).
A. E. Martell and R. M. Smith,Critical Stability Constants, Volume: Amino Acids, Plenum Press, New York and London, 1974, p. 201 and 283.
R. M. Naik and P. C. Nigam,Transition Met. Chem. (in press).
N. Gupta, P. C. Nigam, R. M. Naik (communicated).
D. G. Lambert and M. M. Jones,J. Am. Chem. Soc., 88, 4615 (1966).
K. J. Laidler,Chemical Kinetics, 2nd Edit., TMH Edition, New York, 1973, p. 220.
J. H. Espenson and S. G. Walenuk Jr.,Inorg. Chem., 11, 2035 (1972).
S. K. Upadhyay and M. C. Agarwal,J. Ind. Chem. Soc., 58, 871 (1981).
A. I. Vogel,Textbook of Macro and Micro Qualitative Inorganic Analysis, Longman Green and Co., London, 4th Edit., 1954, pp. 348, 349.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Naik, R.M., Nigam, P.C. Kinetics and mechanism of pentacyanohydroxoferrate(III) formation from mono(aminocarboxylato)iron(III) complexes. Transition Met Chem 10, 227–232 (1985). https://doi.org/10.1007/BF00692634
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00692634