Abstract
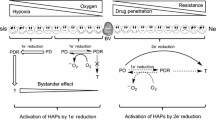
Existing hypoxia-selective cytotoxins (HSCs) are designed to kill only the hypoxic subpopulation in tumours, and to be used in conjunction with other therapies (e.g., radiation). A new class of drugs, hypoxia-activated prodrugs of diffusible cytotoxins (HPDCs) are proposed. These are designed to exploit, rather than merely deal with, tumour hypoxia, by releasing diffusible cytotoxins on bioreduction in hypoxic regions. Such diffusible cytotoxins are required to be much more cytotoxic than the parent prodrug, to be sufficiently stable (half lives from 0.1 to 10min) to allow them to diffuse up to 200 µm from the hypoxic regions, and to be equally effective against all major tumour cell subpopulations, including non-cycling cells. Nitrogen mustards, which show little cell cycle specificity, which kill cells by a well-understood mechanism (DNA cross-links), and which have stabilities and reactivities able to be predictably controlled by structural variations, are proposed as suitable candidates fur such diffusible cytotoxins. Design parameters for two classes of potential HPDCs are discussed; nitro-deactivated aromatic mustards, and cobalt (III) complex-deactivated aliphatic mustards. Examples of both classes show greater cell-killing activity against intact compared with dissociated multi-cellular spheroids. This suggests they may indeed function as HPDCs, by penetrating to the hypoxic core of the spheroid and there releasing potent cytotoxins which diffuse out to kill surrounding cells at lower oxygen tensions.
Similar content being viewed by others
References
Petry E: Zur Keuntnis des Bedingungen der Biologischen Wirkung der Rontgenstrahlen. Biochem Zeitschr 135: 353, 1923
Hill RP: Experimental radiotherapy. In: Tannock IF, Hill RP (eds) The Basic Science of Oncology (ed 2nd). McGraw-Hill, New York, 1992, pp 276–301
Henk JM, Smith CW: Radiotherapy and hyperbaric oxygen in head and neck cancer. Lancet: 104–105, 1977
Overgaard J, Hansen HS, Andersen AP, Hjelm-Hansen M, Jorgensen K, Sandberg E, Bertelsen A, Hammer R, Petersen M: Misonidazole combined with split course radiotherapy in treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys 16: 1065–1068, 1989
Bush RS, Jenkin RDT, Allt WEC, Beale FA, Bean H, Dembo AJ, Pringle JF: Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer 37: 302–306, 1978
Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH: Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 14: 831–838, 1988
Wilson WR: Tumour hypoxia: challenges for cancer chemotherapy. In: Waring MJ, Ponder BAJ (eds) Cancer Biology and Medicine (vol 3). Kluwer Academic Publishers, Lancaster, 1992, pp 87–131
Keyes SR, Rockwell S, Sartorelli AC: Porfiromycin as a bioreductive alkylating agent with selective toxicity to hypoxic EMT6 tumor cellsin vivo andin vitro. Cancer Res 45: 3642–3645, 1985
Adams GE, Stratford IJ, Edwards HS, Bremner JCM, Cole S: Bioreductive drugs as post-irradiation sensitizers: comparison of dual function agents with SR 4233 and the mitomycin C analogue EO9. Int J Radiat Oncol Biol Phys 22: 717–720, 1992
Cole S, Stratford IJ, Bowler J, Nolan J, Wright EG, Lorimore SA, Adams GE: Oral (po) dosing with RSU 1069 or RB 6145 maintains their potency as hypoxic cell radiosensitizers and cytotoxins but reduces systemic toxicity compared with parenteral (ip) administration in mice. Int J Radiat Oncol Biol Phys 21: 387–395, 1991
Zeman EM, Hirst VK, Lemmon MJ, Brown JM: Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother Oncol 12: 209–218, 1988
Chaplin DJ, Olive PL, Durand RE: Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res 47: 597–601, 1987
Brown JM, Koong A: Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst 83: 178–185, 1991
Brown JM: Targeting bioreductive drugs to tumours: is it necessary to manipulate blood flow? Int J Rad Biol 60: 231–236, 1991
Brown JM, Lemmon MJ: Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res 50: 7745–7749, 1990
Kallman RF: Reoxygenation and repopulation in irradiated tumors. Front Radiat Ther Oncol 22: 30–49, 1988
Bump EA, Taylor YC, Brown JM: Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res 43: 997–1002, 1983
Adams G, Barnes D, Du Boulay C, Loutit J, Cole S, Sheldon P, Stratford I, Van Den Aardweg G, Hopewek J, White R, Kneen G, Nethersell A, Edwards C: Induction of hypoxia in normal and malignant tissue by changing the oxygen affinity of haemoglobin - implications for therapy. Int J Radiat Oncol Biol Phys 12: 1299–1302, 1986
Chaplin DJ: Potentiation of RSU-1069 tumour cytotoxicity by 5-hydroxytryptamine (5-HT). Br J Cancer 54: 727–731, 1986
Brown JM: Exploitation of bioreductive agents with vasoactive drugs. In: Fielden EM, Fowler JF, Hendry JH, Scott D (eds) Radiation research: Proceedings of the 8th International Congress of Radiation Research. Taylor and Francis, London, 1987, pp 719–724
Chaplin DJ: Hypoxia-targetted chemotherapy: a role for vasoactive drugs. In: Fielden EM, Fowler JF, Hendry JH, Scott D (eds) Radiation research: Proceedings of the 8th International Congress of Radiation Research. Taylor and Francis, London, 1987, pp 579–585
Chaplin DJ, Acker B: The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069. Evidence for therapeutic gain. Int J Radiat Oncol Biol Phys 13: 579–585, 1987
Stratford IJ, Adams GE, Gooden J, Howells N: Induction of tumour hyoxia post-irradiation: a method for increasing the sensitizing efficiency of misonidazole and RSU 1069in vivo. Int J Rad Biol 55: 411–422, 1989
Chaplin DJ, Peters CE, Horsman RM, Trotter M: Drug induced perturbations in tumor blood flow: therapeutic potential and possible limitations. Radiother Oncol 20: 93–101, 1991
Stratford IJ, Godden J, Howells N, Embling P, Adams GE: Manipulation of tumour oxygenation by hydralazine increases the potency of bioreductive radiosensitizers and enhances the effect of melphalan in experimental tumours. In: Fielden EM, Fowler JF, Hendry JH, Scott D (eds) Radiation Research: Proceedings of the 8th International Congress of Radiation Research. Taylor and Francis, London, 1987, pp 737–742
Rowell NP, Flower MA, McCready VR, Cronin V, Horwich A: The effects of single dose oral hydralazine on blood flow through human lung tumors. Radiother Oncol 18: 283–292, 1990
Cole S, Robbins L: Manipulation of oxygenation in a human tumour xenograft with BW12C or hydralazine:: Effects on responses to radiation and to the bioreductive toxicity of misonidazole and RSU 1069. Radiother Oncol 16: 235–243, 1989
Cole S, Stratford IJ, Adams GE: Manipulations of radiobiological hypoxia in a human melanoma xenograft to exploit the bioreductive cytotoxicity of RSU 1069. Int J Rad Biol 56: 587–591, 1989
Lemmon MJ, Brown JM: Hydralazine does not increase hypoxia in tumors growing in preirradiated tissue. Int J Radiat Oncol Biol Phys 21: 1435–1440, 1991
Zwi LJ, Baguley BC, Gavin JB, Wilson WR: Blood flow failure as a major determinant in the antitumour action of flavone acetic acid (NSC 347512). J Natl Cancer Inst 81: 1005–1013, 1989
Sun J, Brown JM: Enhancement of the antitumor effect of flavone acetic acid by the bioreductive cytotoxic drug SR 4233 in a murine carcinoma. Cancer Res 49: 5664–5670, 1989
Mahadevan V, Malik STA, Meager A, Fiers W, Lewis GP, Hart IR: Role of tumor necrosis factor in flavone acetic acidinduced tumor vasculature shutdown. Cancer Res 50: 5537–5542, 1990
Thomsen LL, Ching Lai-M, Baguley BB: Evidence for the production of nitric oxide by activated macrophages treated with the antitumor agents flavone-8-acetic acid and xanthenone-4-acetic acid. Cancer Res 50: 6966–6970, 1990
Stone HB, Michinton A, Lemmon M, Menke D, Brown JM: Pharmacological modification of tumor blood flow: lack of correlation between alteration of mean arterial blood pressure and changes in tumor perfusion. Int J Radiat Oncol Biol Phys 22: 79–86, 1991
Edwards HS, Bremner JCM, Stratford IJ: Induction of hypoxia in the KHT sarcoma by tumour necrosis factor and flavone acetic acid. Int J Rad Biol 59: 419–432, 1991
Edwards HS, Bremner JCM, Stratford IJ: Induction of tumour hypoxia by FAA and TNF: interaction with bioreductive drugs. Int J Rad Biol 60: 373–377, 1991
Kerr DJ, Maughan T, Newlands E, Rustin G, Bleehen NM, Lewis C: Phase II trials of flavone acetic acid in advanced malignant melanoma and colorectal carcinoma. Br J Cancer 60: 104–106, 1989
Braunschweiger PG, Jones SA, Johnson CS, Furmanski P: Potentiation of mitomycin C and porfiromycin antitumor activity in solid tumor models by recombinant human interleukin 1α1,2. Cancer Res 51: 5454–5460, 1991
Braunschweiger PG, Basrur VS: The effect of interleukin-1alpha onin vivo radiosensitization by SR 4233 in RIF-1 tumors. 40th Annual Meeting, Radiation Research Society (abstract): 19, 1992
Taylor YC, Rauth AM: Differences in the toxicity and metabolism of the 2-nitroimidazole misonidazole (RO-07-0582) in HeLa and Chinese hamster ovary cells. Cancer Res 38: 2745–2752, 1978
Whitmore GF, Gulyas S, Varghese AJ: Sensitizing and toxicity properties of misonidazole and its derivatives. Br J Cancer 37 (Suppl III): 115–119, 1978
Olive PL, Durand RE: Activation of radiosensitizers by hypoxic cells. Br J Cancer 37: 124–128, 1978
Durand RE, Olive PL: Evaluation of nitroheterocyclic radiosensitizers using spheroids. Adv Radiat Biol 9: 75–107, 1981
Brown JM: Cytotoxic effects of the hypoxic cell radiosensitizer Ro 7-0582 to tumor cellsin vivo. Radiat Res 72: 469–486, 1977
Berube LR, Harasiewicz K, Foster FS, Dobrowsky E, Sherar MD, Rauth AM: Use of a high frequency ultrasound microscope to image the action of 2-nitroimidazoles in multicellular spheroids. Br J Cancer 65: 633–640, 1992
Franko AJ, Koch CJ: Binding of misonidazole to V79 spheroids and fragments of Dunning rat prostatic and human colon carcinomasin vitro: diffusion of oxygen and reactive metabolites. Int J Radiat Oncol Biol Phys 10: 1333–1336, 1984
Marshall RS, Rauth AM: Oxygen and exposure kinetics as factors influencing the cytotoxicity of porfiromycin, a mitomycin C analogue, in Chinese hamster ovary cells. Cancer Res 48: 5655–5659, 1988
Rauth AM, Marshall RS: Mechanisms of activation of Mitomycin C and AZQ in aerobic and hypoxic mammalian cells. In: Adams GE (ed) Selective activation of drugs by redox processes. Plenum Press, New York, 1990, pp 113–123
Humm JL: Dosimetric aspects of radiolabelled antibodies for tumour therapy. J Nuclear Medicine 27: 1490–1497, 1986
Bagshawe KD: Antibody-directed enzyme/prodrug therapy (ADEPT). Biochem Soc Trans 18: 750–752, 1990
Hoban PR, Walton MI, Robson CN, Godden J, Stratford IJ, Workman P, Harris AL, Hickson ID: Decreased NADPH: cytochrome P-450 reductase activity and impaired drug activation in a mammalian cell line resistant to mitomycin C under aerobic but not hypoxic conditions. Cancer Res 50: 4692–4697, 1990
Tannock IF, Rotin D: Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 49: 4373–4384, 1989
Chaplin DJ, Acker MD, Olive PL: Potentiation of the tumor cytotoxicity of melphalan by vasodilating agents. Int J Radiat Oncol Biol Phys 16: 1131–1135, 1989
Palmer BD, Wilson WR, Pullen SM, Denny WA: Hypoxiaselective antitumor agents. 3. Relationships between structure and cytotoxicity against cultured tumour cells for substituted N,N-bis(2-chloroethyl) anilines. J Med Chem 33: 112–121, 1990
Brophy GT, Sladek NE: Influence of pH on the cytotoxic activity of chlorambucil. Biochem Pharmacol 32: 79–84, 1983
Skarsgard LD, Chaplin DJ, Wilson DJ, Skwarchuk MW, Vinczan A, Kristl J: The effect of hypoxia and low pH on the cytotoxicity of chlorambucil. Int J Radiat Oncol Biol Phys 22: 737–741, 1992
Tannock I: Cell kinetics and chemotherapy: A critical review. Cancer Treat Rep 62: 1117–1133, 1978
Lewis DFV: Molecular orbital calculations on tumor-inhibitory phenyl aziridines: QSARs. Xenobiotica 19: 341–356, 1989
Wilman DEV, Connors AT: Molecular structure and antitumour activity of alkylating agents. In: Neidle S, Waring MJ (eds) Molecular Aspects of Anticancer Drug Action. McMillan, London, 1983, pp 1–34
Chapman JD, Franko AJ, Sharplin J: A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer 43: 546–550, 1981
Urtasun RC, Koch CJ, Franko AJ, Raleigh JA, Chapman JD: A novel technique for measuring human tissue pO2 at the cellular level. Br J Cancer 54: 453–457, 1986
Raleigh JA, Miler GG, Franko AJ, Koch CJ, Fuciarelli AF, Kelly DA: Fluorescence immunohistochemical detection of hypoxic cells in spheroids and tumours. Br J Cancer 56: 395–396, 1987
Hodgkiss RJ, Jones G, Long A, Parrick J, Smith KA, Stratford MRL, Wilson GD: Flow cytometric evaluation of hypoxic cells in solid experimental tumours using fluorescence immunodetection. Br J Cancer 63: 119–125, 1991
Zeman EM, Massenburg GS, Cline JM, Thrall DE, Raleigh JA: Proliferative and oxygenation status in spontaneous canine tumors: an immunocytochemical approach. In: Chapman JD, Dewey WC, Whitmore GF (eds) Radiation Research, a Twentieth-Century Perspective (vol 1). Academic Press, Toronto, 1991, p 251
Franko AJ, Koch CJ, Boisvert DPJ: Distribution of misonidazole adducts in 9L gliosarcoma tumors and spheroids: implications for oxygen distribution. Cancer Res 52: 3831–3837, 1992
Thorndyke C, Meeker BE, Thomas G, Lakey WH, McPhee MS, Chapman JD: The radiation sensitivities of R3327-H and R3327-AT rat prostate adenocarcinomas. Journal of Urology 134: 191–198, 1985
Koh Wui-J, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, Krohn KA, Griffin TW: Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 22: 199–212, 1991
Parliament MB, Chapman JD, Urtasun RC, McEwan AJ, Golberg L, Mercer JR, Mannan RH, Wiebe LI: Non-invasive assessment of human tumour hypoxia with123I-iodoazomycin arabinoside: preliminary report of a clinical study. Br J Cancer 65: 90–95, 1992
Boag JW: Oxygen diffusion and oxygen depletion problems in radiobiology. In: Ebert M, Howard A (eds) Current Topics in Radiation Research (vol 5). North-Holland, Amsterdam and London, 1969, pp 141–195
Wilson WR, Denny WA: DNA-binding nitroheterocycles as hypoxia-selective cytotoxins: In: Dewey WC, Edington M, Fry RJM, Hall EJ, Whitmore GF (eds) Radiation Research, a Twentieth-Century Perspective (vol 2). Academic Press, Toronto, 1992, pp 796–801
Panthananickal A, Hansch C, Leo A: Structure-activity relationships in antitumor aniline mustards. J Med Chem 21: 1626, 1978
Palmer BD, Wilson WR, Cliffe S, Denny WA: Hypoxia-selective antitumor agents. 5. Synthesis of water-soluble nitroaniline mustards with selective cytotoxicity for hypoxic mammalian cells. J Med Chem 35: 3214–3222, 1992
Knox RJ, Friedlos F, Jarman M, Roberts JJ: A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem Pharmacol 37: 4661–4669, 1988
Knox RJ, Boland MP, Friedlos F, Coles B, Southan C, Roberts JJ: The nitroreductase enzyme in Walker cells that activates 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) to 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide is a form of NAD(P)H dehydrogenase (quinone) (EC 1.6.99.2). Biochem Pharmacol 37: 4671–4677, 1988
Knox RJ, Friedlos F, Marchbank T, Roberts JJ: Bioactivation of CB 1954: reaction of the active 4-hydroxylamino derivative with thioesters to form the ultimate DNA-DNA interstrand crosslinking species. Biochem Pharmacol 42: 1691–1697, 1991
Friedlos F, Quinn J, Knox RJ, Roberts JJ: The properties of total adducts and interstrand crosslinks in the DNA of cells treated with CB 1954: exceptional frequency and stability of the crosslink. Biochem Pharmacol 43: 1249–1254, 1992
Stratford IJ, Williamson C, Hoe S, Adams GE: Radiosensitizing and cytotoxicity studies with CB 1954 (2,4-dinitro-5-aziridinylbenzamide). Radiat Res 88: 502–509, 1981
Cliffe S, Thompson KM, Palmer BD, van Zijl P, Wilson WR: Identification of the major hypoxic metabolites of CB 1954 and its nitrogen mustard analogue, SN 23862, in Chinese hamster cells. 40th Ann Meeting, Radiation Research Soc p 60 (Abstract)
Atwood JD: Inorganic and organometallic reaction mechanisms. Brooks/Cole, Monterey, 1985, p 87
Hendry P, Ludi A: Structure, reactivity, spectra and redox properties of cobalt (III) hexamines. Adv Inorg Chem 35: 117–198, 1990
Simic M, Lilie J: Kinetics of ammonia detachment from reduced cobalt (III) complexes based on conductometric pulse radiolysis. J Am Chem Soc 96: 291–292, 1974
Eaton DR, O'Reilly A: Oxidation of cobalt (II) amine complexes to mononuclear cobalt (III) complexes by dioxygen. Inorg Chem 26: 4185–4188, 1987
Fallab S, Mitchell PR: Binuclear dioxygen complexes of cobalt. In: Sykes AG (ed) Advances in Inorganic and Bioinorganic Mechanisms. Academic Press, London, 1984, p 326
Jackson TB, Edwards JO: Coordination complexes of ethyleneimine with Co(III), Cr(III), Pd(II), Pt(II) and Pt(IV). Inorg Chem 1: 398–401, 1962
Ware DW, Siim BG, Robinson KG, Denny WA, Brothers PJ, Clark GR: Synthesis and characterization of aziridine complexes of cobalt (III) and chromium (III) designed as hypoxia-selective cytotoxins. X-ray crystal structure of trans[Co(Az)2(NO2)2]Br2.2H2O.LiBr. Inorg Chem 30: 3750–3757, 1991
Teicher BA, Abrams MJ, Rosbe KW, Herman TS: Cytotoxicity, radiosensitization, antitumor activity and interaction with hyperthermia of a Co(III) mustard complex. Cancer Res 50: 6971–6975, 1990
Shinohara N, Lilie J, Simic MG: Kinetics and mechanism of ligand dissociation of cobalt (II)-polyamine complexes in aqueous solution. Inorg Chem 16: 2809–2813, 1977
Ware DW, Wilson WR, Denny WA, Rickard CEF: Design and synthesis of cobalt (III) nitrogen mustard complexes as hypoxia selective cytotoxins. The X-ray crystal structure of bis(3-chloro-2,4-pentanedionato) (RS-N,N′-bis(2-chloroethyl)-ethylenediamine) cobalt (III) perchlorate, [Co (Clacac)2(BCE)]ClO4. J Chem Soc Chem Commun: 1171-1173, 1991
Franko AJ, Sutherland RM: Radiation survival of cells from spheroids grown in different oxygen concentrations. Radiat Res 79: 454–467, 1979
Wilson WR, van Zijl P, Denny WA: Bis-bioreductive agents as hypoxia-selective cytotoxins: nitracrine N-oxide. Int J Radiat Oncol Biol Phys 22: 693–696, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Denny, W.A., Wilson, W.R. Bioreducible mustards: a paradigm for hypoxia-selective prodrugs of diffusible cytotoxins (HPDCs). Cancer Metast Rev 12, 135–151 (1993). https://doi.org/10.1007/BF00689806
Issue Date:
DOI: https://doi.org/10.1007/BF00689806