Summary
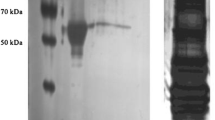
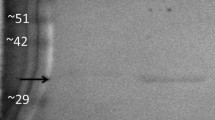
Pyruvate kinases from flight muscle and fat body of the cockroach,Periplaneta americana, were purified to homogeneity. The two tissues contained different forms of the enzyme which were separable by starch gel electrophoresis and isoelectric focusing (pI=5.75 for flight muscle and 6.15 for fat body). Both enzymes had molecular weights of 235,000±20,000.
Flight muscle pyruvate kinase displayed Michaelis-Menten kinetics with respect to both ADP and P-enolpyruvate withK m values of 0.27 and 0.04 mM, respectively.K m for Mg2+ was 0.60 mM andK a for K+ was 15 mM. The enzyme was weakly inhibitied by four compounds, ATP, arginine-P,l-alanine and citrate with apparentK i values of 3.5, 15, 20 and 24 mM, respectively. Competitive inhibition by 3 mM ATP or 10 mM arginine-P raised theK m for P-enolpyruvate to 0.067 or 0.057 mM. Fructose-1,6-P2 did not activate the enzyme but reversed inhibitions by ATP and arginine-P.
Fat body pyruvate kinase showed sigmoidal kinetics with respect to P-enolpyruvate with S0.5=0.32 mM andn H=1.43.K m values for ADP and Mg2+ were 0.30 and 0.80 mM, respectively with aK a for K+ of 10 mM. ATP andl-alanine were inhibitors of the enzyme; 2 mM ATP raised S0.5 for P-enolpyruvate to 0.48 mM while 3 mMl-alanine increased S0.5 to 0.84 mM. Neither citrate nor arginine-P inhibited the enzyme but citrate affected the enzyme by reversingl-alanine inhibition. Fat body pyruvate kinase was strongly activated by fructose-1,6-P2 with an apparentK a of 1.5 M. Fructose-1,6-P2 at 0.1 mM reduced S0.5 for P-enolpyruvate to 0.05 mM andn H to 1.0.
Flight muscle and fat body pyruvate kinases from the cockroach show properties analogous to those of the muscle and liver forms of mammalian pyruvate kinase. Fat body pyruvate kinase is suited for on-off function in a tissue with a gluconeogenic capacity. Strong allosteric control with a feed-forward activation by fructose-1,6-P2 is key to coordinating enzyme function with glycolytic rate. The function of flight muscle pyruvate kinase in energy production during flight is aided by a lowK m for P-enolpyruvate, weak inhibitor effects by high energy phosphates and deinhibition of these effects by fructose-1,6-P2.
Similar content being viewed by others
References
Bailey E (1975) Biochemistry of insect flight. 2. Fuel supply. In: Candy DJ, Kilby BA (eds) Insect biochemistry and function. Chapman and Hall, London, pp 89–176
Bailey E, Walker PR (1969) A comparison of the properties of the pyruvate kinases of the fat body and flight muscle of the adult male desert locust. Biochem J 111:359–364
Beis I, Newsholme EA (1975) The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J 152:23–32
Cornish-Bowden A (1979) Fundamentals of enzyme kinetics. Butterworths, London, pp 28–30
Dando PR (1970) Megrim populations in the English channel and approaches: lactate dehydrogenase and glycerol-3-P dehydrogenase. J Mar Biol Assoc UK 50:801–818
Downer RGH, Parker GH (1979) Glycogen utilisation during flight in the American cockroach,Periplaneta americana. Comp Biochem Physiol A 64:29–32
Giles IG, Poat PC, Munday KA (1977) An investigation of the interactions of the allosteric modifiers by pyruvate kinase with the enzyme fromCarcinus maenas hepatopancreas. Biochem J 165:97–105
Guderley HE, Hochachka PW (1977) Gluconeogenic control adaptations inCancer magister: Hypodermal pyruvate kinase, an enzyme with high- and low-affinity states. Arch Biochem Biophys 182:465–477
Guderley HE, Storey KB, Fields JHA, Hochachka PW (1976) Catalytic and regulatory properties of pyruvate kinase isozymes from octopus mantle muscle and liver. Can J Zool 54:863–870
Hall ER, Cottam GL (1978) Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem 9:785–793
Hoffmann KH (1975) Pyruvate kinase from muscle and fat body of the house cricket,Acheta domesticus L.: Purification and catalytic studies. J Comp Physiol 104:59–69
Hoffmann KH (1977) The regulatory role of muscle pyruvate kinase in carbohydrate metabolism of invertebrates: a comparative study in catalytic properties of enzymes isolated fromTubifex tubifex (Oligochaeta) andTenebrio molitor (Coleoptera). Physiol Zool 50:142–155
Hoffmann KH (1981) Inhibition ofTubifex pyruvate kinase by storage phosphagens and adenosine triphosphate. Comp Biochem Physiol 70B:77–83
Ibsen KH (1977) Interrelationships and functions of the pyruvate kinase isozymes and their variant forms: A review. Cancer Res 37:341–353
Kemp RG (1973) Inhibition of muscle pyruvate kinase by creatine phosphate. J Biol Chem 248:3963–3967
Lesicki A (1976) Characteristic of isozymes of pyruvate kinase isolated from some crayfishOrconectes limosus Raf. (Crustacea: Decapoda) tissues. Comp Biochem Physiol B 55:273–277
Mustafa T, Hochachka PW (1971) Catalytic and regulatory properties of pyruvate kinase in tissues of a marine bivalve. J Biol Chem 246:3196–3203
Newsholme EA, Sugden PH, Opie LH (1970) The apparent inhibition of phosphofructokinase by reduced nicotinamideadenine dinucleotide: a problem of coupled enzyme assays. Biochem J 119:787–789
Scopes RK (1968) Methods for starch gel electrophoresis of sarcoplasmic proteins. Biochem J 107:139–150
Steele JE (1981) The role of carbohydrate metabolism in physiological function. In: Downer RGH (ed) Energy metabolism in insects. Plenum Press, New York, pp 101–133
Storey KB (1977) Purification and characterization of arginine kinase from the mantle muscle of the squid,Symplectotheutis oualaniensis. Arch Biochem Biophys 179:518–526
Storey KB (1981) Effects of arginine phosphate and octopine on glycolytic enzyme activities fromSepia officinalis mantle muscle. J Comp Physiol 142:501–507
Storey KB (1983) Regulation of cockroach flight muscle phosphofructokinase by fructose 2,6-bisphosphate. Role in the activation of muscle metabolism during flight. FEBS Lett 161:265–268
Storey KB, Hochachka PW (1975) Squid muscle pyruvate kinase: control properties in a tissue with an active α-GP cycle. Comp Biochem Physiol 52B:187–191
Waddell W (1956) A simple UV spectrophotometric method for the determination of protein. J Lab Clin Med 48:311–314
Weber K, Osborn M (1969) The reliability of molecular weight determinations by dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412
Wieser W, Lackner R (1977) Inhibition of the pyruvate kinase ofHelix pomatia L. by phospho-l-arginine. FEBS Lett 80:299–302
Zwaan A de (1972) Pyruvate kinase in muscle extracts of sea mussel,Mytilus edulis L. Comp Biochem Physiol B 42:7–14
Zwaan A de, Ebberink RHM (1978) Apparent inhibition of pyruvate kinase by arginine phosphate. FEBS Lett 89:301–303
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Storey, K.B. Kinetic and regulatory properties of pyruvate kinase isozymes from flight muscle and fat body of the cockroach,Periplaneta americana . J Comp Physiol B 155, 339–345 (1985). https://doi.org/10.1007/BF00687476
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687476