Summary
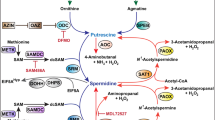
N-Alkylated polyamine analogues have been shown to exert antiproliferative effects in several tumor models, with the bis-ethyl derivatives exerting the greatest suppression of polyamines by virtue of down-regulation of the polyamine biosynthetic enzymes. Pancreatic adenocarcinoma presents a challenge both clinically and experimentally due to its inherent resistance to conventional therapy, which results in its having the worst 5-year survival rate of all cancers. We have previously shown thatN 1,N 12-bis(ethyl)spermine (BESPM) is much more potent than the polyamine enzyme inhibitor α-difluoromethylornithine (DFMO) against pancreatic adenocarcinoma cell lines. In the present study, we compared the biochemical and antiproliferative effects of twoN-alkylated polyamine analogues,N 1,N 14-bis(ethyl)homospermine (BEHSPM) andN 1,N 11-bis(ethyl)norspermine (BENSPM) in two human pancreatic ductal adenocarcinoma cell lines, PANC-1 (poorly differentiated) and BxPC-3 (moderately well-differentiated), and in the WD PaCa (well-differentiated ductal) hamster cell line. BENSPM displayed greater antiproliferative activity in the human pancreatic cancer cell lines, whereas BEHSPM was more potent in the hamster cell line. Both BEHSPM and BENSPM suppress the activity of the major biosynthetic enzymes ornithine decarboxylase andS-adenosylmethionine decarboxylase. However, the induction of polyamine depletion in the human cell lines was only modest for BENSPM and minimal for BEHSPM, which suggests that the substantial antiproliferative activity of these analogues may result from mechanisms other than polyamine depletion. The somewhat greater polyamine depletion seen following treatment with BENSPM is thought to result from its striking induction of spermidine/spermineN 1-acetyltransferase. The biochemical and antiproliferative activity of BENSPM makes it an attractive agent for further preclinical and clinical development, especially in pancreatic cancer.
Similar content being viewed by others
References
Basu HS, Feuerstein BG, Deen DF, Lubich WP, Bergeron RJ, Samejima K, Marton LJ (1989) Correlation between the effects of polyamine analogues on DNA conformation and cell growth. Cancer Res 49: 5591–5597
Bergeron RJ, Garlich JR, Stolowich NJ (1984) Reagents for the step-wise functionalization of spermidine, homospermidine, and bis-(3-aminopropyl)-amine. J Org Chem 49:2997–3001
Bergeron RJ, Hawthorne TR, Vinson JRT, Beck DE Jr, Ingeno MJ (1989) Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res 49: 2959–2964
Reference deleted
Bolkenius FN, Seiler N (1981) Acetyl-derivatives as intermediates in polyamine catabolism. Int J Biochem 13: 287–292
Byers TL, Pegg AE (1990) Regulation of polyamine transport in Chinese hamster ovary cells. J Cell Physiol 143: 460–467
Casero RA Jr, Go B, Theiss W, Smith J, Baylin SB, Luk GD (1987) Cytotoxic response of the relatively difluoromethylornithine-resistant human lung tumor cell line NCI H157 to the polyamine analogueN 1,N 8-bis(ethyl)spermidine. Cancer Res 47:3964–3967
Casero RA Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR (1989) Differential induction of spermidine/spermineN 1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res 49: 3829–3833
Casero RA, Celano P, Ervin SJ, Wiest L, Pegg AE (1991) High specific induction of spermidine/spermineN 1-acetyltransferase in a human large-cell lung carcinoma. Biochem J 270: 615–620
Chang BK (1983) Comparison of in vitro methods for assessing cytotoxic activity against two pancreatic adenocarcinoma cell lines. Cancer Res 43: 3147–3149
Chang BK (1983) Differential sensitivity of pancreatic adenocarcinoma cell lines to chemotherapeutic agents in culture. Cancer Treat Rep 57: 355–361
Chang BK, Gutman R (1982) Chemotherapy of pancreatic adenocarcinoma: initial report on two transplantable models in the Syrian hamster. Cancer Res 42: 2666–2670
Chang BK, Fredericks W, Rustum YM, Baker RM (1988) Levels of P-glycoprotein in pancreatic cancer cell lines: relation to inherent drug resistance (abstract 443). Proc Am Soc Clin Oncol 7: 115
Chang BK, Libby PR, Bergeron RJ, Porter CW (1988) Modulation of polyamine biosynthesis and transport by oncogene transfection. Biochem Biophys Res Commun 157: 264–270
Chang BK, Porter CW, Bergeron RJ (1991) Cellular responses to polyamine analogues and inhibitors in human pancreatic adenocarcinoma cell lines. J Cell Pharmacol 2: 133–137
Chou J, Chou T-C (1986) Dose-effect analysis with microcomputers (Version 2.0). (Elsevier-BIOSOFT, Cambridge, England)
Chou T-C, Talalay P (1984) Quantitative analysis of dose-effect relationship: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22: 27–55
Della Ragione F, Pegg AE (1982) Purification and characterization of spermidine/spermineN 1-acetyltransferase from rat liver. Biochemistry 21: 6152–6158
Gudjonsson B, Livstone EM, Spiro HM (1978) Cancer of the pancreas: diagnostic accuracy and survival statistics. Cancer 42: 2494–2506
Hölttä E (1977) Oxidation of spermidine and spermine in rat liver; purification and properties of polyamine oxidase. Biochemistry 16: 91–100
Kallio A, Sëppanen P, Alhonen-Hongisto L, Jänne J (1983) Modulation of the tissue disposition of methylglyoxal bis(guanylhydrazone) in mice by polyamine depletion and by polyamine administration. Cancer Res 43: 324–327
Kramer DL, Bergeron RJ, Porter CW (1991) Effects of polyamine analogs on spermidine and spermine uptake (abstract 2404). Proc Am Assoc Cancer Res 32: 404
Kupchik HZ, Reisfeld RA, Go VLW (1982) National Pancreatic Cancer Project workshop on pancreatic tumor markers. Dig Dis Sci 27: 65–72
Libby PR (1978) Calf liver nuclearN-acetyl transferases. J Biol Chem 253: 233–237
Libby PR, Henderson MA, Bergeron RJ, Porter CW (1989) Major increase in sperimidine/spermine-N 1-acetyltransferase activity by spermine analogs and their relationship to polyamine depletion and growth inhibition of L1210 cells. Cancer Res 49: 6229–6231
Lieber M, Mazzetta JA, Nelson-Rees W, Kaplan M, Todaro G (1975) Establishment of a continuous tumor-cell line (PANC-1) from a human carcinoma of the exocrine pancreas. Int J Cancer 15: 741–747
Loor R, Nowak NJ, Manzo ML, Douglass HO, Chu TM (1982) Use of pancreas-specific antigen in immunodiagnosis of pancreatic cancer. Clin Lab Med 2: 567–578
O'Brien TG (1976) The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res 36: 2644–2653
Pakala R, Kreisel M, Bachrach U (1988) Polyamine metabolism and interconversion in NIH 3T3 andras-transfected NIH 3T3 cells. Cancer Res 48: 3336–3340
Pegg AE, McCann PP (1982) Polyamine metabolism and function. Am J Physiol 243: C212-C221
Pegg AE, Poso H (1983)S-Adenosylmethionine decarboxylase (rat liver). In: Tabor H, Tabor CW (eds) Methods in enzymology, vol 94. (Academic Press, San Diego) pp 234–239
Pegg AE, Seely JE (1983) Ornithine decarboxylase (mouse kidney). In: Tabor H, Tabor CW (eds) Methods in enzymology, vol 94. (Academic Press, San Diego) pp 158–166
Pegg AE, Wechter R, Pakala R, Bergeron RJ (1989) Effect ofN 1,N 12-bis(ethyl)spermine and related compounds on growth and polyamine acetylation, content, and excretion in human colon tumor cells. J Biol Chem 264: 20
Pegg AE, Pakala R, Bergeron RJ (1990) Induction of spermidine/spermineN 1-acetyltransferase activity in Chinese-hamster ovary cells byN 1,N 11-bis(ethyl)norspermine and related compounds. Biochem J 267: 331–338
Porter CW, Bergeron RJ (1988) Enzyme regulation as an approach to interference with polyamine biosynthesis — an alternative to enzyme inhibition. Adv Enzyme Regul 27: 57–79
Porter CW, Cavanaugh PF, Stolowich N, Ganis B, Kelly E, Bergeron RJ (1985) Biological properties ofN 4- andN 1,N 8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res 45: 2050–2057
Porter CW, Ganis B, Vinson T, Marton LJ, Kramer DL, Bergeron RJ (1986) Comparison and characterization of growth inhibition in L1210 cells by α-difluoromethylornithine, an inhibitor of ornithine decarboxylase, andN 1,N 8-bis(ethyl)spermidine, an apparent regulator of the enzyme. Cancer Res 46: 6279–6285
Porter CW, Berger FG, Pegg AE, Ganis B, Bergeron RJ (1987) Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogueN 1,N 8-bis(ethyl)spermidine. Biochem J 242: 433–440
Porter CW, McManis J, Casero RA, Bergeron RJ (1987) Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res 47: 2821–2825
Porter CW, Pegg AE, Ganis B, Madhabala R, Bergeron RJ (1990) Combined regulation of ornithine andS-adenosylmethionine decarboxylases by spermine and the spermine analogueN 1,N 12-bis(ethyl)spermine. Biochem J 268: 207–212
Porter CW, Ganis B, Libby PR, Bergeron RJ (1991) Correlations between polyamine analogue-induced increases in spermidine/spermineN 1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res 51: 3715–3720
Scalabrino G, Poso H, Hölttä E, Hannonen P, Kallio A, Jänne J (1978) Synthesis and accumulation of polyamines in rat liver during chemical carcinogenesis. Int J Cancer 21: 239–245
Seiler N (1987) Inhibition of enzymes oxidizing polyamines. In: McCann PP, Pegg AE, Sjoerdsma A (eds) Inhibition of polyamine metabolism: biological significance and basis for new therapies. Academic Press, San Diego, pp 49–77
Seiler N, Koch-Weser J, Knödgen B, Richards W, Tardif C, Bolkenius FN, Schechter P, Tell G, Mamont P, Fozard J, Bachrach U, Grosshaus E (1981) The significance of acetylation in the urinary excretion of polyamines. In: Calderera CM, Zappia V, Bachrach U (eds) Advances in polyamine research, vol 3. Raven Press, New York, pp 197–211
Takano S, Matsushima M, Erturk E, Bryan GT (1981) Early induction of rat colonic epithelial ornithine andS-adenosyl-l-methionine decarboxylase activities byN-methyl-N′-nitrosoguanidine or bile salts. Cancer Res 41: 624–628
Author information
Authors and Affiliations
Additional information
This investigation was supported by grant CH-468 from the American Cancer Society, by grant CA-37606 from the National Cancer Institute, Department of Health and Human Services, and by the Department of Veterans Affairs Medical Research Service
Rights and permissions
About this article
Cite this article
Chang, B.K., Bergeron, R.J., Porter, C.W. et al. Regulatory and antiproliferative effects ofN-alkylated polyamine analogues in human and hamster pancreatic adenocarcinoma cell lines. Cancer Chemother. Pharmacol. 30, 183–188 (1992). https://doi.org/10.1007/BF00686309
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686309