Summary
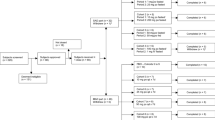
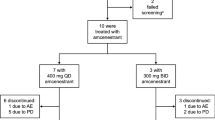
Toremifene is an antiestrogen that binds strongly to estrogen receptors (ER). A total of 19 previously treated postmenopausal women with metastatic breast cancer whose performance status was good and whose ER status was positive or unknown were studied to determine the maximum tolerated dose of toremifene. Cohorts of patients received 200, 300, or 400 mg/m2 p.o. daily until relapse or unacceptable toxicity had occurred. Nausea, vomiting, and dizziness were dose-related. Three of five patients receiving 400 mg/m2 experienced moderate or severe vomiting and another developed reversible disorientation and hallucinations. Mild sweating, peripheral edema, vaginal discharge, and hot flushes were encountered at all doses. Reversible corneal pigmentation was identified in seven cases but was not of clinical importance. The pharmacokinetics of toremifene was studied weekly and in detail on day 42 using a high-performance liquid chromatographic (HPLC) assay that identified the parent compound and three active metabolites,N-desmethyltoremifene, (deaminohydroxy)toremifene, and didemethyltoremifene. Steady state was achieved at 1–3 weeks. The toremifene area under the curve and the maximal concentration were dose-dependent at high doses. The recommended phase II dose is 300 mg/m2 p.o. daily.
Similar content being viewed by others
References
Anttila M, Valavaara R, Kivinen S, Maenpaa J (1990) Pharmacokinetics of toremifene. J Steroid Biochem 36:249–252
Castanon Romo MC, Santander JU, Arez O, Chavez Dominguez R (1989) Amiodarone keratopathy. A prospective longitudinal study. Arch Inst Cardiol Mex 59:245–250
Ebbs SR, Roberts JV, Baum M (1987) Alternative mechanism of action of “antioestrogens” in breast cancer. Lancet II:621
Hietanen T, Baltina D, Johansson R, Numminen S, Hakala T, Helle L, Valavaara R (1990) High dose toremifene (240 mg daily) is effective as first line hormonal treatment in advanced breast cancer. An on-going phase II multicenter Finnish-Latvian co-operative study. Breast Cancer Res Treat 16 [Suppl]:S37-S40
Hyatt RH, Sinha B, Vallon A, Bailey RJ, Martin A (1988) Noncardiac side-effects of long-term oral amiodarone in the eldery. Age Ageing 17:116–122
Kaiser-Kupfer MI, Lippman ME (1978) Tamoxifen retinopathy. Cancer Treat Rep 62:315–320
Kallio S, Kangas L, Blanco G, Johansson R, Karjalainen A, Perila M, Pippo I, Sundquist H, Sodervall M, Toivola R (1986) A new triphenylethylene compound, FC-1157a: I. Hormonal effects. Cancer Chemother Pharmacol 17:103–108
Kangas L (1990) Biochemical and pharmacological effects of toremifene metabolites. Cancer Chemother Pharmacol 27:8–12
Kangas L, Nieminen AL, Blanco G, Gronroos M, Kallio S, Karjalainen A, Perila M, Sodervall M, Toivola R (1986) A new triphenylethylene compound, FC-1157a: II. Antitumor effects. Cancer Chemother Pharmacol 17:109–113
Kennedy BJ, Tibetts DM, Nathanson IT, Aub C (1953) Hypercalcemia, a complication of hormone therapy of advanced breast cancer. Cancer Res 13:445–450
Kiviner M, Maenpaa J (1986) Effect of toremifene on clinical hematological and hormonal parameters in different dose levels. Phase I study (abstract 2994) Proceedings, International Cancer Congress, Budapest, August 21–27 (1986), p 778
Kohler PC, Hamm JT, Wiebe VJ, DeGregorio MW, Shemano I, Tormey DC (1990) Phase I study of the tolerance and pharmacokinetics of toremifene in patients with cancer. Breast Cancer Res Treat 16 [Suppl]:S19-S26
Miller AB, Hoogstaten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Patterson JS, Baum M (1978) Safety of tamoxifen (letter). Lancet I:105
Robinson SP, Jordan VC (1989) Antiestrogenic action of toremifene on hormone-dependent,-independent and heterogeneous breast tumor growth in the athymic mouse. Cancer Res 49:1758–1762
Robinson SP, Mauel DA, Jordan VC (1988) Antitumor actions of toremifene in the 7,12-dimethylbenzanthracene (DMBA)-induced rat mammary tumor model. Eur J Cancer Clin Oncol 24:1817–1821
Robustelli della Cuna G, Pavesi L, Preti P, Baroni M (1988) High dose of medroxyprogesterone acetate in breast cancer: controlled studies. Adv Clin Oncol 1:45–46
Schnipper LE, Come SE (1987) Hypercalcaemia in breast cancer. In: Harris JR, Henderson IC, Hellman S, Kinne DW (eds) Breast diseases. J. B. Lippincott, Philadelphia, pp 552–556
Sedlacek SM, Horwitz KB (1984) The role of progestins and progesterone receptors in the treatment of breast cancer. Steroids 44: 467–484
Sipila H, Nanto V, Kangas L, Anttila M, Halme T (1988) Binding of toremifene to human serum proteins. Pharmacol Toxicol 63:62–64
Tominaga T, Abe O, Izuo M, Nomura Y (1990) A phase I study of toremifene. Breast Cancer Res Treat 16 [Suppl]:S27-S29
Valavaara R (1990) Phase II trials with toremifene in advanced breast cancer: a review. Breast Cancer Res Treat 16 [Suppl]: S31-S35
Valavaara R, Pyrhonen S, Heikkinen M, Rissanen P, Blanco G, Tholix E, Nordman E, Taskinen P, Holsti L, Hajba A (1988) Toremifene, a new antiestrogenic compound, for treatment of advanced breast cancer. Phase II study. Eur J Cancer Clin Oncol 24: 785–790
Valentin-Opran A, Eilon G, Saez S, Mundy GR (1985) Estrogens and antiestrogens stimulate release of bone resorbing activity by cultured breast cancer cells. J Clin Invest 75:726–731
Webster LK, Crinis NA, Stokes KH, Bishop JF (1991) High-performance liquid chromatographic method for the determination of toremifene and its major human metabolites. J Chromatogr 565: 482–487
Wiebe VJ, Benz CC, Shemano I, Cadman TB, DeGregorio MW (1990) Pharmacokinetics of toremifene and its metabolites in patients with advanced breast cancer. Cancer Chemother Pharmacol 25:247–251
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from Farmitalia
Rights and permissions
About this article
Cite this article
Bishop, J., Murray, R., Webster, L. et al. Phase I clinical and pharmacokinetics study of high-dose toremifene in postmenopausal patients with advanced breast cancer. Cancer Chemother. Pharmacol. 30, 174–178 (1992). https://doi.org/10.1007/BF00686307
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686307