Summary
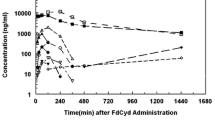
N-(5-[N-(3,4-Dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl)-N-methylamino]-2-thenoyl)-l-glutamic acid (ICI D1694) is an analogue of the thymidylate synthase inhibitorN 10-propargyl-5,8-dideazafolic acid (CB3717). CB3717 was found to be an active anticancer agent in early clinical studies, but its use was limited by its relative insolubility at physiological pH. ICI D1694 has been shown to be a more active anticancer agent than CB 3717 in model systems, and it is devoid of the acute renal toxicity associated with the administration of the latter drug to mice. In the present study, the pharmacokinetics of ICI D1694 were studied in both mice and rats using reverse-phase HPLC. In rats, ICI D1694 clearance (CL) conformed to a two-compartment open model and was rapid (CL=10.7 ml min−1 kg−1,t1/2β=30 min). Excretion was mainly biliary (65% of the delivered dose in 4 h vs 12% in urine) in the rat following a 100-mg/kg i.v. bolus. A high degree of protein binding was seen in rat plasma (≥90% over the range of 20–100 μm). In mice, ICI D1694CL=27 ml min−1 kg−1 andt1/2β=30 min following 100 mg/kg i.v., which was significantly faster than CB3717 clearance (CL=6 ml min−1 kg−1,t1/2β=93 min). ICI D1694 was fully bioavailable following i.p. administration (AUC=3.73 mg ml−1 min i.v. 4.03 mg ml−1 min i.p.), but its bioavailability following oral administration appeared to be low (approximately 10%–20%). Tissue distribution and excretion studies in mice suggested that biliary excretion predominated, confirming the results obtained in rats. Following an i.v. dose of 500 mg/kg ICI D1694 in mice, drug was detectable at 24h, suggesting the presence of a third phase of plasma clearance. The initial HPLC assay could not detect this third phase following a dose of 100 mg/kg; hence, a more sensitive assay was developed that includes a solid-phase extraction step. The latter assay was used to define the third phase of ICI D1694 clearance in mice, and preliminary studies demonstrated a terminal half-life of 6.5±2.7 h.
Similar content being viewed by others
References
Calvert AH, Bondy PK, Harrap KR (1977) Some observations on the human pharmacology of methotrexate. Cancer Treat Rep 61 (9):1647–1656
Calvert AH, Alison DL, Harland SJ, Robinson BA, Jackman AL, Jones TR, Newell DR, Siddick ZH, Wiltshaw E, McElwain TJ, Smith IE, Harrap KR (1986) A phase I evaluation of the quinazoline antifolate thymidylate synthase inhibitor,N 10-propargyl-5,8-dideazafolic acid, CB3717. J Clin Oncol 4 (8):1245–1252
Harrap KR, Jackman AL, Newell DR, Taylor GA, Hughes LR, Calvert AH (1989) Thyrnidylate synthase, a target for anticancer drug design. Adv Enzyme Regul 29:161–179
Jackman AL, Taylor GA, O'Connor BM, Bishop JA, Moran RG, Calvert AH (1990) Activity of the thymidylate synthase inhibitor 2-desamino-N 10-propargyl-5,8-dideazafolic acid and related compounds in murine (L1210) and human (W1L2) systems in vitro and in L1210 in vivo. Cancer Res 50:5212–5218
Jackman AL, Taylor GA, Bishop JA, O'Connor BM, Bisset G, Hughes LR, Moran RG, Calvert AH (1990) Biochemical aspects of a new thymidylate synthase inhibitor, ICI D1694. Proc Am Assoc Cancer Res 31:342
Jackman AL, Marsham PR, Moran RG, Kimbell R, O'Connor BM, Hughes LR, Calvert AH (1991) Thymidylate synthase inhibitors: the in vitro activity of a series of heterocyclic benzoyl ring modified 2-desamino-2-methyl-N 10-substituted-5,8-dideazafolates. Adv Enzyme Regul 31:13–27
Jackson RC, Jackman AL, Calvert AH (1983) Biochemical effects of a quinazoline inhibitor of thymidylate synthetase. CB3717, on human lymphoblastoid cells. Biochem Pharmacol 32:3783–3790
Jodrell DI, Newell DR, Calvete JA, Stephens TC, Calvert AH (1990) Pharmacokinetic and toxicity studies with the novel quinazoline thymidylate synthase inhibitor, ICI D1694 Proc Am Assoc Cancer Res 31:341
Jones TR, Calvert AH, Jackman AL, Brown SJ, Jones M, Harrap KR (1981) A potent antitumour quinazoline inhibitor of thymidylate synthetase: synthesis, biological properties and therapeutic results in mice. Eur J Cancer 17:11–19
Jones TR, Thornton TJ, Flinn A, Jackman AL, Newell DR, Calvert AH (1989) Quinazoline antifolates inhibiting thymidylate synthase: 2-desamino derivatives with enhanced solubility and potency. J Med Chem 32:847–852
Macarol V, Morris TQ, Baker KJ, Bradley SE (1970) Hydrocortisone choleresis in the dog. J Clin Invest 49:1714–1723
Newell DR (1984) The role of pharmacokinetic studies in the development of selective cancer chemotherapy. PhD Thesis, University of London
Newell DR, Alison DL, Calvert AH, Harrap KR, Jarman M, Jones TR, Manteuffel-Cymborowska M, O'Connor P (1986) Pharmacokinetics of the thymidylate synthase inhibitorN 10-propargyl-5,8-dideazafolic acid (CB3717) in the mouse. Cancer Treat Rep 70 (8):971–979
Newell DR, Maxwell RJ, Bisset GMF, Jodrell DI, Griffiths JR (1990) Pharmacokinetic studies with the antifolateC 2-desamino-C 2-methyl-N 10-propargyl-2′-trifluoromethyl-5,8-dideazafolic acid (CB3988) in mice and rats using in vivo [19F]-NMR spectroscopy. Br J Cancer 62 (5):766–772
Shaw H, Capel I, Heath T (1972) Effect of ethacrynic acid on bile formation in sheep, dogs, rats, guinea pigs and rabbits. J Pharmacol Exp Ther 182:27–33
Steinberg SE, Campbell CL, Bleyer WA, Hillman RS (1982) Enterohepatic circulation of methotrexate in rats in vivo. Cancer Res 42:1279–1282
Stephens TC, Calvete JA, Janes D, Waterman SE, Valcaccia BE, Hughes LR, Calvert AH (1990) Antitumour activity of a new thymidylate synthase inhibitor, ICI D1694. Proc Am Assoc Cancer Res 31:342
Valerino DM, Johns DG, Zaharko DS, Oliverio VT (1972) Studies of the metabolism of methotrexate by intestinal flora: 1. The identification and study of biological properties of the metabolite 4-amino-4-deoxy-N 10-methylpteroic acid. Biochem Pharmacol 21:821–831
Wagner JG (1975) Fundamentals of clinical pharmacokinetics. Drug Intelligence Publications, pp 82–83
Author information
Authors and Affiliations
Additional information
These studies were supported by the UK Cancer Research Campaign and the British Technology Group
Rights and permissions
About this article
Cite this article
Jodrell, D.I., Newell, D.R., Gibson, W. et al. The pharmacokinetics of the quinazoline antifolate ICID 1694 in mice and rats. Cancer Chemother. Pharmacol. 28, 331–338 (1991). https://doi.org/10.1007/BF00685685
Issue Date:
DOI: https://doi.org/10.1007/BF00685685