Abstract
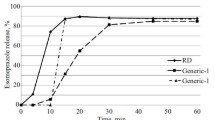
Etoposide is a widely used cytotoxic drug that requires complex formulation for both the i.v. and oral preparation to ensure drug stability. Data on the stability of the i.v. formulation when diluted in infusion fluids are contradictory, and there is little information on the stability of the oral preparation in gastric or intestinal fluids. The stability of both i.v. and oral etoposide was therefore evaluated in the present investigation. The stability of the i.v. preparation was investigated across a range of concentrations in infusion fluids, being determined by regular sampling for highperformance liquid chromatography (HPLC) analysis and by visual inspection. The stability of the oral preparation was studied in both artificial gastric and intestinal fluids, again with regular sampling for HPLC analysis, and the influence of pH, concentration and the addition of ethanol and bile salts on oral stability was determined. The i.v. preparation showed a marked decrease in stability with increasing drug concentration, but stability was additionally reduced in i.v. bags regularly sampled with a syringe and needle as compared with bags that were inspected visually only (minimal stability in sampled bags, 24 h at 0.5 mg/ml and 5 h at 1.0 mg/ml, as compared with 10 days and 18 h at the respective concentrations in unsampled bags). Stability was also greater at room temperature, 20–23°C, as compared with 8–12°C. Loss of stability was indicated by a decrease in etoposide concentration (measured by HPLC) and the appearance of a fine white precipitate, shown to be pure etoposide. Importantly, the appearance of precipitate was as sensitive as a specific HPLC assay in detecting loss of stability and was in many cases apparent when the etoposide concentration was within 5% of the starting concentration. The oral formulation also showed a marked concentration-dependent decrease in stability in artificial intestinal fluid at pH 7.5 (percentage of etoposide in solution after 2 h at 0.5, 1.0, 1.5 and 2.0 mg/ml, 94±2%, 80±5%, 68±13% and 41±9%, respectively). There was no concentration effect on stability in gastric fluid at pH 3.0, although stability was much greater at pH 3 and pH 5 as compared with pH 1 or in intestinal fluid at pH 7.5. Stability in artificial intestinal fluid, pH 7.5, was also significantly improved by the addition of the bile salt sodium tauroglycocholate (2 mg/ml) at etoposide concentrations of 1 (P<0.0001) and 2 mg/ml (P<0.0001) and by the addition of ethanol (10%, v/v) at etoposide levels of 1 (P<0.001) and 2 mg/ml (P<0.001). These studies clearly demonstrate the concentration-dependent stability of both the i.v. and the oral formulation of etoposide, that the appearance of precipitate is a sensitive indicator of loss of stability in i.v. fluids, and that stability in artificial intestinal fluid can be modulated by the use of other agents.
Similar content being viewed by others
References
Adams PS, Haines- Nutt, RF, Rowlands CG (1985) Stability of selected cytotoxics when used by continuous infusion at low doses by home based patients (meeting abstract). EORTC symposium on continuous infusion chemotherapy, March 1985 Brussels, Abstr 9
Adams PS, Haines-Nutt, RF, Bradford, E, Palmer A, Rowland CG (1987) Pharmaceutical aspects of home infusion therapy for cancer patients. Pharm J 238:476–478
Arnold AM (1979) Podophyllotoxin derivative VP16-213. Cancer Chemother Pharmacol 3:71–80
Beijnen, JH, Holthuis JJM, Kerkdijk HG, Houwen OA van der, Paalman AC, Bult A, Underberg WJ (1988) Degradation kinetics of etoposide in aqueous solution. Int J Pharm 41:169–178
Beijnen JH, Beijnen BA, Dubbelman AC, Gijn R van, Underberg WJ (1991) Chemical and physical stability of etoposide and teniposide in commonly used infusion fluids. J Parenter Sci Technol 45:108–112
Bristol-Laboratories (1980) Product development research report. Bristol-Laboratory, Syracuse, New York
Bristol-Myers (1981) Vepesid (etoposide). Current clinical experience. Bristol-Myers, New York
Canetta RP, Hilgard J, Florentine S, Bedogni P, Lenaz L (1982) Current development of podophyllotoxins. Cancer Chemother Pharmacol 7:93–98
D'Incalci M, Erba E, Vaghi M, Morasca L (1982) In vitro cytotoxicty of VP16 on primary tumour and metastasis of Lewis lung carcinoma. Eur J Cancer Clin Oncol 18:377–380
Floor BJ, Klein AE, Muhammad N, Ross D (1985) Stability indicating liquid chromatographic determination of etoposide and benzyl alcohol in injectable formulations. J Pharm Sci 74: 197–200
Guyton AC (1981) Secretory function of the alimentary tract. In: Textbook of medical physiology. W.B. Saunders, Philadelphia, pp 809–811
Harvey VJ, Joel SP, Johnston A, Slevin ML (1985) High-performance liquid chromatography of etoposide in plasma and urine. J Chromatogr 339:419–423
Harvey VJ, Slevin ML, Joel SP, Smythe MM, Johnston A, Wrigley PF (1985) Variable bioavailability following repeated oral doses of etoposide. Eur J Cancer Clin Oncol 21:1315–1319
Harvey VJ, Slevin ML, Joel SP, Johnston A, Wrigley PF (1986) The effect of dose on the bioavailability of oral etoposide. Cancer Chemother Pharmacol 16:178–181.
Joel SP, Clark PI, Maclean MC, and Slevin ML (1989) The stability of the intravenous preparation of etoposide in isotonic fluids (meeting abstract). Proc Am Assoc Cancer Res, Vol. 30:A972
Maanen J van, Vries J de, Pappie D, Akker E van den, Lafleur VM, Retel J, Greef J van der, Pinedo HM (1987) Cytochrome P-450-mediatedO-demethylation: a route in the metabolic activation of etoposide (VP-16-213). Cancer Res 47:4658–4662
McLeod HF, Relling MV (1992) Stability of etoposide solution for oral use. Am J Hosp Pharm 49:2785
Rideout JM, Ayres DC, Lim CK, Peters TJ (1984) Determination of etoposide (VP16-213) and teniposide (VM-26) in serum by high performance liquid chromatography with electrochemical detection. J Pharmacol Biomed Anal 2:125–128
Sandoz (1969) US patent 3 524 844 1976. Chem Abst 70:79340A
Schurgers N, Blaey CJ de, Crommelin DJA (1985) Absorption of etoposide (VP16-213) from the small intestine of the rat. The potential role of mucus as an absorption rate limiting barrier. Pharm Res 4:162–165
Seargeant LE, Kobrinsky NL, Sus CJ, Nazeravich DR (1987) In vitro stability and compatibility of daunorubicin, cytarabine, and etoposide. Cancer Treat Rep 71:1189–1192
Shah JC, Chen JR, Chow D (1989) Preformulation study of etoposide: identification of physicochemical characteristics responsible for the low and erratic oral bioavailability of etoposide. Pharm Res 6:408–412
Stewart CF, Hampton EM (1989) Stability of cisplatin and etoposide in intravenous admixtures. Am J Hosp Pharm 46:1400–1404
Underberg WJM, Kerkdijk HG, Holthuis JJM, Beijnen JH (1985) Analysis and degradation kinetics of etoposide (VP16-213) in aqueous solution. Pharm Weekbl Sci 7:291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joel, S.P., Clark, P.I. & Slevin, M.L. Stability of the i.v. and oral formulations of etoposide in solution. Cancer Chemother. Pharmacol. 37, 117–124 (1995). https://doi.org/10.1007/BF00685638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685638