Summary
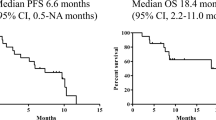
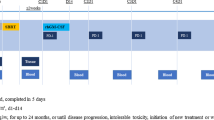
A total of 36 patients with advanced non-small-cell lung cancer (NSCLC) were treated with a combination of 5-day continuous i.v. infusion of cisplatin (25 mg/m2 daily), bolus infusion of vindesine (3 mg/m2) on days 1 and 8, and s.c. injection of recombinant human granulocyte-colony-stimulating factor (2 μg/kg daily) on days 6–21. Treatment was repeated every 3–4 weeks. Responding patients with stage IIIA or IIIB disease received chest radiation therapy (50–60 Gy) after this treatment. One complete response and 23 partial responses were observed, for an overall response rate of 66.7% (24/36; 95% confidence limits, 51.3%–82.1%). The median duration of response was 5.7 months and the median overall survival was 10.1 months. WHO grade 3 or 4 leukopenia and neutropenia occurred in 22 (61%) and 27 (75%) patients, respectively, but the mean duration of leukopenia (<2,000/mm3) and neutropenia (<1,000/mm3) was 3.4 and 3.5 days, respectively, and there was no instance of lifethreatening infection. Thrombocytopenia and anemia of grade 3 or 4 occurred in 28% and 36% of our subjects, respectively. Grade 2 nausea and vomiting occurred in 47% of the patients. Elevated serum creatinine levels (>1.5 mg/dl) were observed in 3 (8%) of the 36 patients. One patient died of acute renal faialure induced by hemorrhage of a gastric ulcer. This regimen is effective in the treatment of NSCLC and further studies of this combination are warranted.
Similar content being viewed by others
References
Belliveau JF, Posner MR, Ferrari L, Crabtree GW, Cummings FJ, Wiemann MC, O'Leary GP Jr, Griffin H, Phaneuf MA, O'Rourke A, Calabresi P (1986) Cisplatin administered as a continuous 5-day infusion: plasma platinum levels and urinary platinum excretion. Cancer Treat Rep 70: 1215
Bunn PA Jr (1989) The expanding role of cisplatin in the treatment of non-small-cell lung cancer. Semin Oncol 16: 10
Carmichael J, Gregor A, Cornbleet MA, Allan SG, Mcintyre MA, Grant IWB, Compton GK, Leonard RCF, Smyth JF (1985) Cis-platinum and vindesine in combination in the treatment of non-small cell lung cancer. Eur J Cancer Clin Oncol 21: 811
Dhindra HM, Valdivieso M, Carr DT, Chiuten DF, Farha CP, Murphy WK, Spitzer G, Umsawasdi T (1985) Randomized trial of three combinations of cisplatin with vindesine and/or VP-16-213 in the treatment of advanced non-small-cell lung cancer. J Clin Oncol 3: 176
Drewinko B, Brown BW, Gottlieb JA (1973) The effect ofcis-diamminedichloroplatinum(II) on cultured human lymphoma cells and its therapeutic implications. Cancer Res 33: 3091
Eguchi K, Sasaki S, Tamura T, Sasaki Y, Shinkai T, Yamada K, Soejima Y, Fukuda M, Fujiwara Y, Kunitou H, Tobinai K, Ohtsu T, Suemasu K, Takaku F, Saijo N (1989) Dose escalation study of recombinant human granulocyte-colony-stimulating factor (KRN 8601) in patients with advanced malignancy. Cancer Res 49: 5221
Einhorn LH, Loeher PJ, Williams S, Meyers S, Gabrys T, Nattan SR, Woodburn R, Drasga R, Songer J, Fisher W, Stephens D, Hui S (1986) Random prospective study of vindensine versus vindesine plus high-dose cisplatin versus vindesine plus cisplatin plus mitomycin C in advanced non-small-cell lung cancer. J Clin Oncol 4: 1037
Fukuoka M, Ariyoshi Y, Furuse K, Niitani H, Motomiya M, Hasegawa K, Tominaga K, Kuriyama T, Yoshida K, Kimura H, Kurita Y, Nakajima S, Nakai J, Ota M, Yamamoto H, Ota K (1990) Effect of recombinant human G-CSF (rG·CSF) in patients with non-small cell lung cancer (NSCLC) treated with combination chemotherapy of mitomycin C, vindesine, and cisplatin (MVP chemotherapy). Biotherapy 4: 1038
Gralla RJ, Casper ES, Kelsen DP, Braun DW Jr, Dukeman ME, Martini N, Young CW, Golbey RB (1981) Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: a randomized trial investigating two dosage schedules. Ann Intern Med 95: 414
Kaplan EL, Meier P (1958) Non-parametric estimation from incomplete observations. J Am Stat Assoc 63: 457
Kris MG, Gralla RJ, Kalman LA, Kelsen DP, Casper ES, Burke MT, Groshen S, Cibas IR, Bagin R, Heelan RT (1985) Randomized trial comparing vindesine plus cisplatin with vinblastine plus cisplatin in patients with non-small cell lung cancer, with an analysis of methods of response assessment. Cancer Treat Rep 69: 387
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50: 163
Matsushima Y, Kanzawa F, Hoshi A, Shimizu E, Nomori H, Sasaki Y, Saijo N (1985) Time-schedule dependency of the inhibiting activity of various anticancer drugs in the clonogenic assay. Cancer Chemother Pharmacol 14: 104
Miller AB, Hoogatraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207
Mori K, Saito Y, Tominaga K (1992) Phase II study of cisplatin continuous infusion plus vindesine in the treatment of non-small-cell lung cancer. Am J Clin Oncol (in press)
Mountain CF (1986) A new international staging system for lung cancer. Chest 89: 225
Saito Y, Mori K, Tominaga K, Yokoi K, Miyazawa N (1990) Phase II study of 5-day continuous infusion ofcis-diamminedichloroplatinum(II) in the treatment of non-small-cell lung cancer. Cancer Chemother Pharmacol 26: 389
Shinkai T, Saijo N, Tominaga K, Eguchi K, Shimizu E, Sasaki Y, Fujita J, Futami H (1985) Comparison of vindesine plus cisplatin or vindesine plus mitomycin in the treatment of advanced non-small cell lung cancer. Cancer Treat Rep 69: 945
Sørensen JB, Clerici M, Hansen HH (1988) Single-agent chemotherapy for advanced adenocarcinoma of the lung. Cancer Chemother Pharmacol 21: 89
Woods RL, Williams CJ, Levi J, Page J, Bell D, Byrne M, Kerestes ZL (1990) A randomized trial of cisplatin and vindesine versus supportive care only in advanced non-small cell lung cancer. Br J Cancer 61: 608
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saito, Y., Mori, K., Tominaga, K. et al. Phase II study of cisplatin as a 5-day continuous infusion with vindesine plus recombinant human granulocyte-colony-stimulating factor in the treatment of advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol. 31, 81–84 (1992). https://doi.org/10.1007/BF00685091
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685091