Abstract
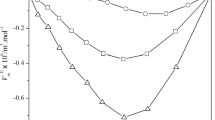
Isothermal compressibilities κT and isobaric thermal expansion coefficients αp have been determined for mixtures of ethylbenzene+n-nonane, +n-decane, and +n-dodecane at 25 and 45°C in the whole range of composition. The excess functions\((\kappa _\nu ^V )^E = - (\partial V^{\text{E}} /\partial p)_{\text{T}} \) and\((\alpha p{\text{V}})^{\text{E}} = (\partial {\text{V}}^{\text{E}} /\partial T)_p \) have been obtained at each measured mole fraction. The first one\( - (\partial {\text{V}}^{\text{E}} /\partial p)_{\text{T}} )\) is zero for ethylbenzene +n-nonane, positive for ethylbenzene +n-decane, and +n-dodecane and increases with chain length n of the n-alkane. The\((\partial {\text{V}}^{\text{E}} /\partial T)_{\text{P}} \) function is positive for the three studied systems and nearly constant with n. Both mixing functions increase slightly with temperature. From this measurement and supplementary literature data of molar heat capacities at constant pressure C P , the isentropic compressibilities κS, the molar heat capacities at constant volume C V and the corresponding mixing functions have been calculated at 25°C. Furthermore, the pressure dependence of excess enthalpy HB,\((\partial H^{\text{E}} /\partial p)_{\text{T}} \) at zero pressure and at 25°C has been obtained from our experimental results of\((\partial V^{\text{E}} /\partial T)_{\text{P}} \) and experimental literature values for excess volume VE.
Similar content being viewed by others
References
A. D. Matilla, E. Aicart, M. Diaz Peña, and G. Tardajos,J. Solution Chem. 17, 000 (1988) (in press).
M. Costas, H. V. Tra, D. Patterson, M. Caceres G. Tardajos and E. Aicart,J. Chem. Soc. Faraday Trans. I 84, 1603 (1988).
E. Aicart, G. Tardajos, and M. Costas,J. Solution Chem. (submitted).
Selected Values of Physical Thermodynamic Properties of Hydrocarbons and Related Compounds, API Research Project 44. Thermodynamic Research Center (Texas A&M University, College Station, Texas 1977).
M. Diaz Peña and M. L. McGlashan,Trans. Faraday Soc. 57, 1511 (1961);
M. Diaz Peña and G. Tardajos,J. Chem. Thermodynamics 10, 19 (1978).
M. Caceres J. M. Arsuaga, and J. Nuñez,Fluid Phase Equilibria 20, 81 (1985).
J. L. Fortier and G. C. Benson,J. Chem. Eng. Data 25, 47 (1980).
J. F. Messerly, G. B. Guthrie, S. S. Todd, and H. L. Finke,J. Chem. Eng. Data 12, 338 (1967).
J. P. E. Grolier, A. Faradjzadeh, and H. V. Keihaian,Thermochim. Acta 53, 157 (1982).
Y. P. Handa, C. J. Halpin, and G. C. Benson,J. Chem. Thermodynn 13, 875 (1981).
J. J. Christensen, J. B. Ott, and J. F. Moellmer,J. Chem. Thermodynn 9, 249 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matilla, A.D., Tardajos, G., Diaz Peña, M. et al. First and second thermodynamic mixing functions of ethylbenzene+n-nonane, +n-decane, and+n-dodecane at 25 and 45°C. J Solution Chem 18, 893–901 (1989). https://doi.org/10.1007/BF00685064
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00685064