Summary
-
1.
Anaerobic and aerobic energy production during and after induced bursts of maximum swimming activity were measured in four size classes of rainbow trout fry at 4, 12 and 20°C.
-
2.
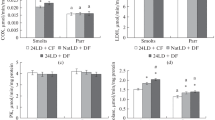
The rate of phosphocreatine (PCr) utilization during burst activity appeared independent of size and temperature. On average the concentration of PCr dropped from 5.8±1.0 to 1.5±0.7 μmol·g−1 within 30 s of stimulation, and decreased only slightly more after an additional 30 sec of stimulation. The concentration of ATP also decreased to 70% of control levels, whereas the concentrations of ADP and AMP remained constant, in agreement with other investigations on vertebrate white muscles. Lactate concentrations did not change during the shorter period of stimulation but from then on increased steadily through the second half of the one minute stimulus and through the recovery period observed (10 min).
-
3.
Five minutes after the end of stimulation phosphocreatine had recovered 67% of the control level whereas the concentration of ATP as well as the energy charge remained low. After ten minutes not only phosphocreatine but also ATP and energy charge had recovered at least 80% of the ground lost during the period of exertion.
-
4.
The following parameters of aerobic energy production were distinguished:Routine rate (\(\dot V_{O_2 } \)r): maintained for several hours before or after induced burst activity;maximum rate (\(\dot V_{O_2 } \)max): attained for a few minutes during and immediately after an induced burst of activity;net recovery \(\dot V_{O_2 } \): amount of oxygen consumed during recovery time minus the amount due to routine rate during this interval.
-
5.
The routine rate of\(\dot V_{O_2 } \) is affected by temperature but less so by the age of the fry. On the other hand,\(\dot V_{O_2 } \)max and net recovery,\(\dot V_{O_2 } \) increased strongly with age at the two lower temperatures. Aerobic scope for activity increases with size and is maximum at 12°C.
-
6.
The age-dependent changes of\(\dot V_{O_2 } \) are discussed in relation to the differentiation of the swimming musculature and to hydrodynamic changes, since between a body length of 1 and 10 cm, the fish move through a range of Reynolds numbers from approximately 102 to 104.
-
7.
It appears, furthermore, that with increasing size, more energy is liberated during the recovery period than would be required for repapying the true oxygen debt. This may be due to an elevated activity during poststimulus excitement.
Similar content being viewed by others
Abbreviations
- PCr :
-
phosphocreatine
- \(\dot V_{O_2 } \)r:
-
routine rate of oxygen consumption
- \(\dot V_{O_2 } \)max:
-
maximum rate of oxygen consumption
References
Beamish FWH (1978) Swimming capacity. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VII. Academic Press, New York, pp 101–189
Bergmeyer HU (1974) Methoden der enzymatischen Analyse. 3. Auflage, Bd 2. Verlag Chemie, Weinheim/Bergstraße
Black EC (1957) Alterations in the blood level of lactic acid in certain salmonid fishes following muscular activity. I. Kamloops trout,Salmo gairdneri. J Fish Res Bd Can 14:117–134
Blake RW (1983) Fish locomotion. Cambridge University Press, Cambridge
Bone Q (1978) Locomotor muscle. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VII. Academic Press, New York, pp 361–424
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Bd Can 21:1183–1226
Brett JR, Groves TDD (1979) Physiological Energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol-VIII. Academic Press, New York, pp 280–352
Chandrasena SI, Hird FJ (1978) Comparative aspects of adenylic acid deaminase and aspartate-2-oxoglutarate aminotransferase. Comp Biochem Physiol 61B:191–194
Childress S (1981) Mechanics of swimming and flying. Cambridge University Press, Cambridge
Dolinin VA (1973) The rate of basal metabolism in fish. J Ichthyol (english translation) 13:430–438
Driedzic WR, Hochachka PW (1976) Control of energy metabolism in carp white muscle. Am J Physiol 230:579–582
Forstner H (1983) An automated multiple-chamber intermittent-flow respirometer. In: Gnaiger E, Forstner H (eds) Polarographic oxygen sensors. Aquatic and physiological applications. Springer, Berlin Heidelberg New York Tokyo, pp 111–126
Forstner H, Hinterleitner, S, Mähr, K, Wieser W (1983) Towards a better definition of ‘metamorphosis’ inCoregonus sp.: biochemical, histological, and physiological data. Can J Fish Aquat Sci 40:1224–1232
Fry FEJ (1947) Effects of the environment on animal activity. Univ Toronto Stud Biol Ser 55:1–62
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VI. Academic Press, New York, pp 1–98
Hagberg JM, Mullin JP, Nagle FJ (1980) Effect of work intensity and duration on recovery O2 J Appl Physiol 48:540–544
Markarewicz W (1969) AMP-aminohydrolase in muscle of elasmobranch fish. Purification procedure and properties of the purified enzyme. Comp Biochem Physiol 29:1–26
Meyer RA, Terjung RL (1979) Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. Am J Physiol 237:C111–118
Nag AC, Nursall JR (1972) Histogenesis of white and red fibres of the trunk muscle of a fish,Salmo gairdneri.. Cytobios 6:227–247
Onnen T, Zebe E (1983) Energy metabolism in the tail muscle of the shrimpCrangon crangon during work and subsequent recovery. Comp Biochem Physiol 74A:833–838
Raffin JP, Leray C (1980) Comparative study on AMP deaminase in gill, muscle and blood of fish. Comp Biochem Physiol 67B:533–540
Randall DJ (1970) The circulatory system. In: Hoar WS, Randall DJ (eds) Fish physiology, vol IV. Academic Press, New York, pp 133–172
Vetter RD, Hodson RE (1982) Use of adenylate concentrations and adenylate energy charge as indicators of hypoxic stress in estuarine fish. Can J Fish Aqu Sci 39:535–541
Waarde A, van, Kesbeke F (1981) Regulatory properties of AMP-deaminase from lateral red muscle and dorsal white muscle of goldfish,Carassius auratus L. Comp Biochem Physiol 69B:413–423
Waarde A van, Kesbeke F (1983) Goldfish muscle energy metabolism during electrical stimulation. Comp Biochem Physiol 75B:635–639
Walesby NJ, Johnston IA (1980) Temperature acclimation in brook trout muscle: Adenine nucleotide concentrations, phosphorylation state and adenylate energy charge. J Comp Physiol 139:127–133
Webb PW (1975) Hydrodynamics and energetic of fish propulsion. Bull Fish Res Bd Can 190:159
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wieser, W., Platzer, U. & Hinterleitner, S. Anaerobic and aerobic energy production of young rainbow trout (Salmo gairdneri) during and after bursts of activity. J Comp Physiol B 155, 485–492 (1985). https://doi.org/10.1007/BF00684679
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00684679