Summary
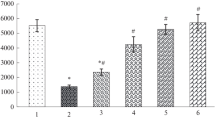
Lactate concentration was measured in the abdominal muscle of the shrimpPalaemon serratus. Rapid and seasonal temperature changes result in an increase of the lactate content of approximately 3–4 fold.
Lactate dehydrogenase from the abdominal muscle exhibits a temperature dependent pyruvate inhibition with pyruvate as substrate.
The kinetic parameters of lactate dehydrogenase fromPalaemon serratus are found to vary during rapid temperature changes: Vmax increases with temperature from 0.06 μmol min−1 (mg protein)−1 at 10°C to 0.28 μmol min−1 (mg protein)−1 at 30°C with lactate as substrate, and from 5.5 μmol min−1 (mg protein)−1 at 10°C to 26.2 μmol min−1 (mg protein)−1 at 30°C, with pyruvate (Table 1). The Hill coefficientn H, decreases with temperature from 2.2 to 1.2 when the pyruvate reduction is examined, but remains near 1.2 when the activity is measured with lactate as substrate (Table 1). The S0.5 values for lactate show a tendency to increase below 30 °C (18.9 mM l−1 at 20 °C) whereas the S0.5 for pyruvate is found to increase greatly with temperature (0.004 mM l−1 at 10 °C and 0.06 mM l−1 at 20 °C).
Long term temperature changes involve variations of lactate dehydrogenase activity leading to inverse thermal compensation (Table 2).
Activation energy (about 56 kJ both with pyruvate and lactate) does not vary during the year, suggesting that temperature adaptation does not induce important catalytic changes (Table 3).
Similar content being viewed by others
Abbreviations
- LDH:
-
lactate dehydrogenase
References
Alexandrov VY (ed) (1977) Cells, molecules and temperature. In: Ecological studies, vol 21. Springer, Berlin Heidelberg New York
Everse J (1975) Enzymic determination of lactic acid. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol XLl/B. Academic Press, New York, pp 41–44
Fitts RH, Holloszy JO (1976) Lactate and contractile force in frog muscle during development of fatigue and recovery. J Physiol 231:430–433
Freed JM (1969) Changes in activity of cytochrome oxidase during adaptation of goldfish to different temperatures. Comp Biochem Physiol 14:651–659
Granata AL, Midrio L, Corsi A (1976) Lactate oxidation by skeletal muscle during sustaining contractionin vivo. Pflügers Arch 366:247–250
Guinier D (1979) Bioinformatique: réalisation d'un sous-système interactif et conversationnel, son application dans l'exploitation des données expérimentales en biologie. Thèse 265, Université Louis Pasteur, Strasbourg, France
Hazel J, Prosser CL (1970) Interpretation of inverse acclimation to temperature. Z Vergl Physiol 67:217–228
Hochachka PW, Somero GN (1971) Biochemical adaptation to the environment. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 6, Academic Press, New York, pp 100–156
Johnston IA, Davison, W., Goldspink G (1975) Adaptations in Mg++ ATPase activity induced by temperature acclimation. FEBS Lett 50:293–295
Lowry DH, Rosebrough NF, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marquart DW (1963) An algorithm for least squares estimation of non linear parameters. J Soc Indust Appl Math 2:431–441
Moon TW, Hochachka PW (1971) Isocitrate dehydrogenase in rainbow trout liver. Biochem J 123:695–705
Precht H, Laudien H, Havsteen B (1973) Concepts of the temperature adaptation of unchanging reaction systems of cold blooded animals. In: Precht H, Christophersen J, Hensel H, Lercher W (eds) Temperature and life. Springer, Berlin Heidelberg New York, pp 302–398
Sahlin K, Harris RC, Nylind B, Haltman E (1976) Lactate content and pH in muscle samples obtained after dynamic exercise. Pflügers Arch 367:143–149
Somero GN (1978) Temperature adaptation of enzymes: biological optimization through structure-function compromises. Annu Rev Ecol Syst 9:1–29
Somero GN, Low PS (1977) Eurytolerant proteins: mechanisms for extending the environmental tolerance range of enzymeligand interactions. Am Nat 3:527–538
Thébault MT, Bernicard A, Lennon JF (1981) Lactate dehydrogenase from the caudal muscle of the shrimpPalaemon serratus: purification and characterization. Comp Biochem Physiol [B] 68:65–70
Traush G (1976) Effect of temperature upon catalytic properties of lactate dehydrogenase in the lobster. Biochem Syst Ecol 4:65–68
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thébault, M.T. Lactate content and lactate dehydrogenase activity inPalaemon serratus abdominal muscle during temperature changes. J Comp Physiol B 154, 85–89 (1984). https://doi.org/10.1007/BF00683220
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00683220