Summary
-
1.
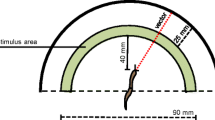
The marine gastropod molluskPleurobranchaea was avoidance conditioned by pairing food stimuli with conditional aversive electric shock. Yoked control specimens received explicitly unpaired food and shock in similar quantities to experimentals. All specimens were tested blind.
-
2.
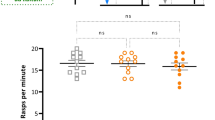
Experimental animals (N = 7) trained against squid homogenate (conditioned stimulus or CS) acquired an aversion to the squid, as evidenced by withdrawal from the CS (active avoidance learning; Fig. 1) and suppression of feeding behavior (passive avoidance learning; Figs. 2 and 3). The acquired aversion persisted for longer than 1 week, and was not displayed by control animals (N = 7). Statistically significant differences between experimentals and controls tested with squid were obtained in ten out of fifteen post-conditioning comparisons.
-
3.
Experimental animals trained against squid homogenate, as well as control specimens in the same paradigm, showed a weak aversion to a homogenate of the sea anemoneCorynactis (Figs. 1–3). The difference between experimentals and controls when tested withCorynactis was statistically significant in only two out of fifteen comparisons, however. It may be concluded that the learned aversion was specific to the CS associated with shock (squid).
-
4.
42 specimens were subjected to modified differential avoidance conditioning, using various combinations of squid,Corynactis and shrimp as the CS+ (the food stimulus paired conditionally with shock) and CS− (the food stimulus not paired with shock). Differential avoidance learning, evidenced by a statiscally significant difference between responses to the CS+ and CS−, was evident in the withdrawal and feeding behaviors of individual animals, individual experiments on small groups of animals and in the aggregate data from all experiments (Figs. 4 and 5).
-
5.
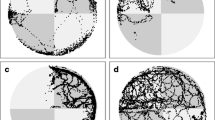
When data were categorized by the combination of stimuli used as the CS+ and CS−, the strongest differential learning was exhibited by the group in whichCorynactis served as the CS+ and squid as the CS− (Figs. 6–10). For certain combinations of food stimuli, differential learning was not obtained.
-
6.
We conclude that the behavioral modification induced by avoidance conditioning ofPleurobran chaea's feeding and withdrawal behaviors is representative of genuine associative learning, and that the learned aversion is specific to the stimulus with which punishment is associated.
Similar content being viewed by others
Abbreviations
- CS :
-
conditioned stimulus
References
Davis, W.J.: Plasticity in the invertebrates. In: Neural mechanisms of learning and memory. Bennett, E.L., Rosenzweig, M.R., (eds.), pp. 430–462. Cambridge, Mass: MIT Press 1975
Davis, W.J., Gillette, R.: Neural correlate of behavioral plasticity in command neurons ofPleurobranchaea. Science199, 801–804 (1978)
Davis, W.J., Mpitsos, G.J.: Behavioral choice and habituation in the marine molluskPleurobranchaea californica MacFarland (Gastropoda. Opisthobranchia). Z. Vergl. Physiol.75, 207–232 (1971)
Davis, W.J., Siegler, M.V.S., Mpitsos, G.J.: Distributed neuronal oscillators and efference copy in the feeding system ofPleurobranchaea. J. Neurophysiol.36, 258–274 (1973)
Davis, W.J., Mpitsos, G.J., Siegler, M.V.S., Pinneo, J.M., Davis, K.B.: Neuronal substrates of behavioral hierarchies and associative learning in the molluskPleurobranchaea. Am. Zoologist14, 1037–1050 (1974a)
Davis, W.J., Mpitsos, G.J., Pinneo, J.M.: The behavioral hierarchy of the molluskPleurobranchaea. I. The dominant position of the feeding behavior. J. Comp. Physiol.90, 207–331 (1974b)
Davis, W.J., Mpitsos, G.H., Pinneo, J.M.: The behavioral hierarchy of the molluskPleurobranchaea. II. Hormonal suppression of feeding associated with egg-laying. J. Comp. Physiol.90, 225–243 (1974c)
Garcia, J., Koelling, R.A.: Relation of cue to consequence in avoidance learning. Psychonom. Sci.4, 123–124 (1966)
Garcia, J., Ervin, F.R., Koelling, R.A.: Learning with prolonged delay of reinforcement. Psychonom. Sci.5, 121–122 (1966)
Gillette, R., Davis, W.J.: The role of the metacerebral giant neuron in the feeding behavior ofPleurobranchaea. J. Comp. Physiol.116, 129–159 (1977)
Gillette, R., Kovac, M.P., Davis, W.J.: Command neurons inPleurobranchaea receive synaptic feedback from the motor network they excite. Science199, 798–801 (1978)
Gillette, R., Gillette, M.U., Davis, W.J.: Action potential broadening and endogenously sustained bursting are substrates of command ability in a feeding neuron ofPleurobranchaea. J. Neurophysiol.43, 669–685 (1980)
Gormezano, I., Moore, J.W.: History and method. In: Learning Processes. Marx, M.M. (ed.), pp. 121–203. Toronto: Macmillan 1969
Kovac, M.P., Davis, W.J.: Behavioral choice: neural mechanisms inPleurobranchaea. Science198, 632–634 (1977)
Kovac, M.P., Davis, W.J.: Neural mechanism underlying behavioral choice inPleurobranchaea. J. Neurophysiol.43, 469–487 (1980)
Lee, R.M.: Conditioning inPleurobranchaea. Science193, 72–73 (1976)
Maes, F.W., Bijpost, S.C.A.: Classical conditioning reveals discrimination of salt taste quality in the blowflyCalllphora vicina. J. Comp. Physiol.133, 53–62 (1979)
Mpitsos, G.J., Collins, S.D.: Learning: Rapid aversive conditioning in the gastropod molluskPleurobranchaea. Science188, 954–957 (1975)
Mpitsos, G.J., Davis, W.J.: Learning: Classical and avoidance conditioning in the molluskPleurobranchaea. Science180, 317–320 (1973)
Mpitsos, G.J., Collins, S.D., McClellan, A.D.: Learning: A model system for physiological studies. Science199, 497–506 (1978)
Nelson, M.C.: Classical conditioning in the blowfly (Phormia regina): Associative and excitatory factors. J. Comp. Physiol. Psychol.77, 353–368 (1971)
Seligman, M.E.P., Hager, J.L. (eds.): Biological Boundaries of Learning. New York: Appleton-Century-Crofts 1972
Siegel, S.: Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill 1956
Siegler, M.V.S.: Motor neurone coordination and sensory modulation in the feeding system of the molluscPleurobranchaea. J. Exp. Biol.71, 27–48 (1977)
Siegler, M.V.S., Mpitsos, G.J., Davis, W.J.: Motor organization and generation of rhythmic feeding output in the buccal ganglion ofPleurobranchaea. J. Neurophysiol.37, 1173–1196 (1974)
Author information
Authors and Affiliations
Additional information
This research was funded by NIH Research Grants MH23254 and NSO9050 to W.J.D. We thank Dr. G. Bicker, Dr. R. Croll and Dr. M.P. Kovac for comments on the manuscript, and Mr. Eugene Matera for technical assistance.
Rights and permissions
About this article
Cite this article
Davis, W.J., Villet, J., Lee, D. et al. Selective and differential avoidance learning in the feeding and withdrawal behavior ofPleuobranchaea californica . J. Comp. Physiol. 138, 157–165 (1980). https://doi.org/10.1007/BF00680439
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00680439