Abstract
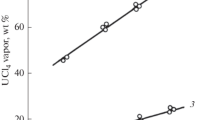
The equilibrium composition of the vapor above thorium nitrate-nitric acid-water mixtures has been studied as a function of the concentrations of thorium nitrate and nitric acid using a transpiration technique. At 25°C, the thorium nitrate concentrations m T ranged from 0.1 to 2.5 molal and the nitric acid concentrations m N from 0.3 to 25 modal. The vapor pressure of the nitric acid was found to increase with increasing thorium nitrate concentration for a constant molality of nitric acid in aqueous solution. At constant m T , the nitric acid vapor pressure was particularly enhanced at low nitric acid concentrations. The water vapor pressures decreased regularly with increasing concentrations of both nitric acid and thorium nitrate. The experimental data were fitted to Scatchard's ion-component model, and to empirical multiparameter functions. From the fitting parameters, and available literature data for the nitric acid-water and thorium nitrate-water systems at 25°C, expressions were calculated for the variation of water and thorium nitrate activities, as functions of the nitric acid and thorium nitrate concentrations, using the Gibbs-Duhem equation. Calculated values for the thorium nitrate activities were strongly dependent on the form of the function originally used to fit the vapor pressure data.
Similar content being viewed by others
References
K. S. Pitzer and J. J. Kim,J. Am. Chem. Soc. 96, 5701 (1974).
R. M. Pytkowicz,Activity Coefficients in Electrolyte Solutions, Vol. 1 and 2, (CRC Press Inc. Boca Raton, Florida, 1979).
M. H. Lietzke and R. W. Stoughton,J. Inorg. Nucl. Chem. 36, 1315 (1974).
R. M. Rush and J. S. Johnson,J. Chem. Thermodyn. 3, 779 (1971).
S. Banerjee, E. Critoph, and R. G. Hart,Can. J. Chem. Eng. 53, 291 (1975).
A. Apelblat, D. Azoulay, and A. Sahar,JCS Faraday I 69, 1618 (1973).
A. Apelblat, D. Azoulay, and A. Sahar,JCS Faraday I 69, 1624 (1973).
A. N. Efimov, M. I. Zhikharev, Yu. P. Zhirnov, and A. Ya. Chilikin,Russ. J. Phys. Chem. (Eng. Trans.) 47, 1208 (1973).
J. R. Ferraro, L. I. Katzin, and G. Gibson,J. Am. Chem. Soc. 76, 909 (1954).
J. R. Hebert and M. W. Lister, unpublished report MX-185 (1945). Results quoted in: W. Morgan, AECL-508 (1958).
A. V. Nikolaev, A. E. Ryabinin, and Yu. A. Afanas'ev,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk (3), 129 (1966).
W. Davis, Jr. and H. J. de Bruin,J. Inorg. Nucl. Chem. 26, 1069 (1964).
A. I. Vogel,A Textbook of Quantitative Inorganic Analysis 3rd. ed., (Longmans, London, 1962), p. 442.
W. Davis, Jr., P. S. Lawson, H. J. de Bruin, and J. Mrochek,J. Phys. Chem. 69, 1904 (1965).
M. A. Yakimov and V. Ya. Mishin,Sov. Radiochem. (Eng. Trans.) 6, 523 (1964).
G. Scatchard, R. M. Rush, and J. S. Johnson,J. Phys. Chem. 74, 3786 (1970).
R. A. Robinson and B. J. Levien,Trans. Proc. Roy. Soc. New Zealand 76, 295 (1947).
K. S. Pitzer and G. Mayorga,J. Phys. Chem. 77, 2300 (1973).
K. S. Pitzer,J. Solution Chem. 4, 249 (1975).
H. A. Kuppers, Dissertation, Rheinish-Westfalischen Technischen Hochschule, Aachen (1964).
C. L. Burdick and E. S. Freed,J. Am. Chem. Soc. 43, 518 (1921).
R. Flatt and F. Benguerel,Helv. Chem. Acta 45, 1765 (1962).
A. K. Covington and J. E. Prue,J. Chem. Soc. 1567 (1957).
F. Hartmann and P. Rosenfeld,Z. Phys. Chem. 164, 377 (1933).
V. B. Parker,Thermal Properties of Aqueous Uni-univalent Electrolytes, U. S. Nat. Bureau of Standards Report NSRDS-NBS 2 (1965).
J. A. Duisman and S. A. Stern,J. Chem. Eng. Data 14, 457 (1969).
G. L. Antipenko, E. S. Beletskaia, and A. G. Krylova,J. Appl. Chem. USSR (Eng. Trans.) 31, 847 (1958).
G. S. Kell, inWater: A Comprehensive Treatise, F. Franks ed., (Plenum Press, New York, 1972), p. 384.
O. Redlich, W. E. Gargrave, and W. D. Krostek,Ind. Eng. Chem. Fundamentals 7, 211 (1968).
R. Haase, K.-H. Ducker, and H. A. Kuppers,Ber. Bunsenges. Physik. Chem. 69, 97 (1965).
L. G. Sillen and A. E. Martell, ‘Stability Constants of Metal-Ion Complexes,’The Chemical Society, Special Publications 17, (London, 1964).
Author information
Authors and Affiliations
Additional information
Issued as AECL-7461.
Rights and permissions
About this article
Cite this article
Lemire, R.J., Brown, C.P. Component activities in the system thorium nitrate-nitric acid-water at 25°C. J Solution Chem 11, 203–220 (1982). https://doi.org/10.1007/BF00667602
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00667602