Abstract
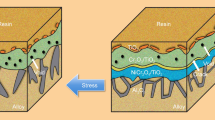
The oxidation of presulphidized Ni-Cr alloys has been studied by taking into account the influence of the two distinct oxidation mechanisms described in part I of this article. Sulphur enters the Cr2O3 scale (in Ni-34Cr alloys) mainly as S2− species, which at high temperatures increases the V‴Cr content, and hence the oxidation kinetics. Sulphur is randomly distributed in the scale, except at the inner oxide-alloy interface, where intergranular microsulphides are analyzed in the oxide-scale zone. In the case of NiO, NiCr2O4, Cr2O3 oxide multilayers (in a Ni-20Cr alloy), sulphur in the S2− state is distributed in the oxide layers or at Si-precipitate interfaces. Such a distribution leads to crack formation, especially during cooling.
Similar content being viewed by others
References
A. Bruckman and S. Mrowec,Werkst. Korros. 25, 502 (1974).
O. H. Hsuch and A. G. Evans,J. Appl Phys. 54, 6672 (1983).
G. Romeo, H. S. Spacil, and W. W. Pasko,J. Electrochem. Soc. 122, 1329 (1975).
R. Lalauze and M. Soustelle,J. Chim. Phys. 70, 1433 (1973).
D. Briggs and M. P. Seah,Practical Surface Analysis (John Wiley & Sons, New York, 1983).
C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder, and G. E. Mullenberg,Handbook of X-Ray Photoelectron Spectroscopy (Perkin Elmer Corp., Eden Prairie, Minnesota, 1979).
J. P. Eberhard, in Doin ed.,Methodes physiques d'étude des minéraux et des matériaux solides (1976), p. 440.
V. I. Nefedou, Y. A. V. Salyn, G. Leonnard, and T. R. Scheibe,J. Electr. Spectro. 10, 121 (1977).
M. Barber, J. A. Connor, M. F. Guest, M. B. Hall, I. H. Hillier, and W. N. G. Meredith,J. Chem. Soc. Far. Disc. 54, 220 (1972).
W. Y. Howng and J. B. Wagner,J Phys. Chem. Solids,30, 191 (1978).
G. Moulin, M. Aucouturier, and P. Lacombe,J. Mat. Sci. 19, 815 (1984).
G. Ben Abderrazik, Thèse de Doctorat des Sciences Physiques, Orsay, France (1986).
R. Grellère and N. Barbouth,Mem. Sc. Rev. Met. 72, 71 (1976).
G. Moulin, A. M. Huntz, G. Ben Abderrazik, and J. M. Rousselet,Proceedings of the 10th International Symposium on Reactivity of Solids, Part A, 235 (Elsevier, 1984).
P. Moulin, A. M. Huntz, and P. Lacombe,Acta Met,27, 1431 (1979).
D. R. Wang, R. Nemoto, and J. B. Wagner,J. Met. Trans. (in press).
P. Pascal,Nouveau traité de chimie minérale (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abderrazik, G.B., Moulin, G. & Huntz, A.M. Relation between impurities and oxide-scale growth mechanisms on Ni-34Cr and Ni-20Cr alloys. II. Influence of sulphur. Oxid Met 33, 237–264 (1990). https://doi.org/10.1007/BF00667416
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00667416