Abstract
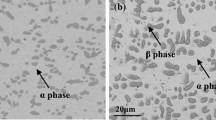
The sulfidation behavior of Co-Mo alloys containing up to 40 wt.% Mo was studied over the temperature range 600–900°C in both 10−2 and 10−4 atm. sulfur vapor. All of the alloys were two-phase, with the alloys containing up to 30Mo consisting of Co3Mo plus solid-solution Co, and the Co-40Mo alloy consisting of the two intermetallic compounds, Co3Mo and Co7Mo6. The sulfide scales which formed were duplex, with an outer layer of cobalt sulfide and a complex, heterophasic inner layer whose phases were both composition- and temperature-dependent. The parabolic rate constant for the sulfidation kinetics decreased with increasing Mo content at all temperatures investigated. Three activation energies, all different from that of pure Co, were observed. Furthermore, Co-30Mo exhibited a kinetics inversion between 800 and 850°C. This inversion was largely the result of the formation of an innermost layer of Co1.62Mo6S8 at the high temperatures. Specifically, the presence of this sulfide in the inner scale caused a significant decrease in the growth rate of the outer layer of cobalt sulfide. In fact, formation of a more compact, innermost layer of Co1.62Mo6S8 at 900°C compared to that at 850°C resulted in a negative activation energy for the growth of the cobalt sulfide in this temperature range. The variation in the activation energies was due to both the duplex nature of the scales which formed and the phase constitution of the inner scale. A simple model has been developed to explain the changes in the activation energies. At 800°C the sulfidation rate of the Co-Mo alloys was essentially the same at the two sulfur pressures studied. The predominant phase in the inner layer of Co-10Mo and Co-20Mo was CoMoS3, while for Co-30Mo and Co-40Mo it was MoS2. However, in the case of the latter alloys, Co1.62Mo3S4 formed in the region of the alloy/scale interface at temperatures 850°C and above. Although the MoS2, which had formed on Co-40Mo, appeared to be a continuous layer, it was in fact found to be relatively nonprotective. Platinummarker experiments revealed the position of the original metal surface to be the interface between the inner and outer scales.
Similar content being viewed by others
References
K. Natesan,Corrosion 41, 646 (1985).
M. Van De Voorde,J. Mat. Sci. Eng. 88, 341 (1987).
W. T. Bakker, R. A. Perkins, and J. Van Liere,Materials Performance 24, 9 (1985).
R. W. Staehler, ed.,Proceedings of the Workshop on Materials Problems and Research Opportunities in Coal Conversion (National Science Foundation, 1974).
F. H. Stott, F. M. F. Chong, and C. A. Sterling,Mat. Sci. Forum 43, 327 (1989).
J. C. Colson, M. Lambertin, and J. P. Larpin, inHigh-Temperature Corrosion, R. A. Rapp, ed. (NACE-6, Houston, Texas, 1983), p. 363.
S. Mrowec and K. Przybylski,High Temp. Mat. Processes 6, 1 (1984).
B. Gleeson, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 209 (1989).
M. F. Chen, D. L. Douglass, and F. Gesmundo,Oxid. Mett. 31, 237 (1989).
M. F. Chen and D. L. Douglass,Oxid. Met. 32, 185 (1989).
R. V. Carter, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 341 (1989).
Ge Wang, R. V. Carter, and D. L. Douglass,Oxid. Met. 32, 273 (1989).
B. Gleeson and D. L. Douglass,Mat. Sci. Engr. A 120, 39 (1989).
K. N. Strafford, G. R. Winstanley, and J. M. Harrison,Werkstoff Korros. 25, 487 (1974).
T. B. Massalski, J. L. Murray, L. H. Benett, and H. Baker,Binary Alloy Phase Diagrams (ASM, 1986).
D. P. Whittle, S. K. Verma, and J. Stringer,Corr. Sci. 13, 247 (1973).
T. Rosenquist,J. Iron Steel Inst. 176, 37 (1954).
G. Hagenbach, P. H. Courty, and B. Delmon,J. Catal. 31, 264 (1973).
Y. Suzuki, T. Uchida, M. Wakihara, and M. Taniguchi,Mat. Sci. Bull. 16, 1085 (1981).
B. Johnson, W. S. Hong, and D. W. Ready,Acta Met. 17, 919 (1983).
J. A. Wilson and A. D. Yoffe,Adv. Phys. 18, 193 (1969).
R. H. Friend and A. D. Yoffe,Adv. Phys. 36, 193 (1987).
A. L. Farragher and P. Cossee,Catal. Proc. Int. Congr. 5th 2, 1301 (1973).
B. S. Lee and R. A. Rapp,J. Electrochem. Soc. 131, 2998 (1984).
Bull. Alloy Phase Diagrams 1, 93 (1980).
K. Yuon,Solid State Commun. 25, 327 (1978).
P. J. Guillevic, O. Bars, and D. Grandjean,Acta Cryst. B32, 1338 (1976).
R. Chevral, M. Sergent, and J. Prigent,Mat. Res. Bull. 9, 1487 (1974).
C. Wagner,Prog. Solid State Chem. 10, 3 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gleeson, B., Douglass, D.L. & Gesmundo, F. A comprehensive investigation of the sulfidation behavior of binary Co-Mo alloys. Oxid Met 33, 425–455 (1990). https://doi.org/10.1007/BF00666808
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00666808