Abstract
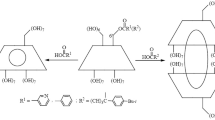
Asymmetric halogenation and hydrohalogenation oftrans-cinnamic acid have been achieved in the microcrystals of cyclodextrin complexes. The bromination of the organic acid in the cavity of β-cyclodextrin gave theerythro-dibromide in 40 % optical yield which was much larger than that from the resolution treatment of the racemic dibromide with β-cyclodextrin and the absolute configuration was opposite in sign. The asymmetric induction in the gas-solid reaction was not due to optical resolution but to the reaction itself which was influenced by the chiral frame of cyclodextrin. The reaction shows the molecular size effect that the acid in the cavity of α-cyclodextrin reacted with smaller hydrogen bromide but did not with larger bromine and chlorine. In contrast, the guest molecule in the wider cavity of β-cyclodextrin reacted with bromine and chlorine as well as hydrogen bromide. The stereospecificities of the gas-solid halogenations of the acid in β-cyclodextrin were similar to those of the both reactions in the solid state and in carbon tetrachloride solution without β-cyclodextrin: bromination of the acid yieldederythro-2, 3-dibromo-3-phenylpropionic acid stereospecifically in 100 % in three different conditions, but chlorination gave an excess ofthreo-2, 3-dichloro-3-phenylpropionic acid to theerythro-isomer in 72∼87 % yields.
Similar content being viewed by others
References
W. P. Jencks,Catalysis in Chemistry and Enzymology, McGraw-Hill, New York, p 282 (1969).
A. R. Fersht,Enzymes Structure and Mechanism, W. H. Freeman & Co., London (1977).
M. L. Bender and M. Komiyama,Cyclodextrin Chemistry, Springer-Verlag, Berlin (1978).
Y. Tanaka, H. Sakuraba, and H. Nakanishi,J. Chem. Soc. Chem. Commun.,1 9 8 3, 947.
J. B. Cohen and C. E. Whiteley,J. Chem. Soc.,79, 1305 (1901).
H. Erlenmeyer,Helv. Chim. Acta,13, 731 (1930).
O. Shimamura and M. Takahashi,Bull. Chem. Soc. Jpn.,22, 60 (1949).
J. A. Riddick and W. B. Bunger, ‘Organic Solvents’, Wiley-Interscience, New York (1970).
J. Szejtli and Zs Budai,Acta Chim. Acad. Sci. Hung.,94, 383 (1977).
K. Uekama, F. Hirayama, K. Esaki, and M. Inoue,Chem. Pharm. Bull.,27, 76 (1979).
A. McKenzie and F. Barrow,J. Chem. Soc.,99, 1910 (1911).
M. L. Poutsma,J. Am. Chem. Soc.,87, 2172 (1965).
M. M. Labes, H. W. Blakeslee, and J. E. Bloor,J. Am. Chem. Soc.,87, 4251 (1965).
R. C. Fahey and H. J. Schneider,J. Am. Chem. Soc.,90, 4429 (1968).
R. E. Buckles, E. A. Hausman, and N. G. Wheeler,J. Am. Chem. Soc.,72, 2494 (1950).
E. Hadjoudis, E. Kariv, and G. M. J. Schmidt,J. Chem. Soc., Perkin Trans. 2, 1056 (1972).
M. C. Cabaleiro and M. D. Johnson,J. Chem. Soc. B, 565 (1967).
G. Schmid and D. C. Garrett,The Chemistry of Functional Groups, Suppl. A. The Chemistry of Double Bonded Functional Groups, Wiley-Interscience, New York (1977).
R. C. Fahey and C. Schubert,J. Am. Chem. Soc.,87, 5172 (1965).
S. Winstein and E. Grunwald,J. Am. Chem. Soc.,70, 828 (1948).
W. R. Vaughan and K. M. Milton,J. Am. Chem. Soc.,74, 5623 (1952).
E. Erlenmeyer, Jr.,Chem. Ber.,39, 788 (1906).
F. Cramer and W. Dietsche,Chem. Ber.,92, 378 (1959).
C. Libermann and H. Finkenbeiner,Chem. Ber.,26, 833 (1893).
A. McKenzie and H. B. P. Humphries,J. Chem. Soc.,97, 121 (1910).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakuraba, H., Nakai, T. & Tanaka, Y. Asymmetric halogenation and hydrohalogenation oftrans-cinnamic acid in crystalline cyclodextrin complexes. Journal of Inclusion Phenomena 2, 829–839 (1984). https://doi.org/10.1007/BF00662252
Issue Date:
DOI: https://doi.org/10.1007/BF00662252