Abstract
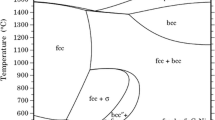
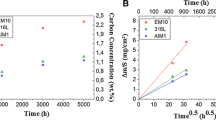
Several commercial and laboratory-cast model austenitic alloys have been exposed in both sulfur-free carburizing environments and also in carburizing atmospheres to which additions of H2S have been made. These studies were concentrated over the temperature range 1223–1323 K at a fixed carbon activity (ac=0.8) with sulfur activities ranging from 2.2×10−12 bar to 1.4×10−9 bar. Under conditions of sulfur adsorption, e.g.,\({\text{p}}_{S_{\text{2}} } \) ≤ 5.5 × 10−11 bar at 1273 K, the blocking of adsorption sites for methane resulted in a transition from the parabolic kinetics observed during sulfur-free carburization to surface controlled linear kinetics. Higher levels of H2S promoted the formation of a surface layer of chromium sulfide which reduced internal carburization but became a problem itself. The role of minor alloying elements has been established and the use of thermodynamic phase stability diagrams in defining the optimum conditions for sulfur inhibition of carburization evaluated.
Similar content being viewed by others
References
G. E. Möller and C. W. Warren,Materials Performance, 27–37, 237 (1981).
H. J. Grabke, G. Tauber, and H. Viefhaus,Scr. Metall. 9, 1181 (1975).
H. J. Grabke, E. M. Petersen, and S. R. Srinivasan,Surf. Sci. 67, 501 (1977).
H. J. Grabke, R. Möller, and A. SchnassProceedings of the International Conference on Behaviour of High Temperature Alloys in Aggressive Environments (The Metal Society, London, 1980), p. 759.
T. A. Ramanarayanan and D. J. Srolovitz,J. Electrochem. Soc. 132, 2268 (1985).
J. M. Harrison, J. F. Norton, R. T. Derricott, and J. B. Marriott,Werkstoff u. Korrosion,30, 785 (1979).
H. E. Bühler and H. P. Hougardy, Atlas der Interferenzschichten—Metallographie (Deutsche Gesellschaft für Metallkunde, Oberursel, West Germany, 1979).
T. B. Pierce, J. W. McMillan, P. F. Peck and I. G. Jones,Nucl. Instrum. Methods,118, 115 (1974).
I. Barin, O. Knacke, and O. Kubaschewski,Thermodynamic Properties of Inorganic Substances and Supplement (Springer-Verlag, Dusseldorf, 1973, 1977).
F. N. Manzandarany and R. D. Pehlke,Metall. Trans. 4, 2067 (1973).
I. Ansara, T. G. Chart, P. Y. Chevalier, K. Hack, G. McHugh, M. H. Rand, and P. J. Spencer, Study Contract between the European Atomic Energy Community and Scientific Group Thermodata (SGTE), 1980, Report no. 18/80/SC.
A. Schnaas and H. J. Grabke,Oxid. Met. 12, 387 (1978).
S. K. Bose and H. J. Grabke,Z. Metallkunde,69, 8 (1978).
T. Wada, H. Wada, J. F. Elliott, and J. Chipman,Metall. Trans. 2, 2199 (1971).
J. F. Elliott and J. Chipman, inChemical Metallurgy of Iron and Steel (Iron and Steel Institute, London, 1973), p. 348.
J. Barnes, J. Corish, F. Franck, and J. F. Norton,Oxid. Met. 24, 85 (1985).
A. W. Cramb, W. R. Graham, and G. R. Belton,Metall. Trans. 9B, 623 (1978).
H. Rau,J. Less-common Metals,55, 205 (1977).
D. J. Young, W. W. Smeltzer, and J. S. Kirkaldy,J. Electrochem. Soc. 120, 1221 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barnes, J., Corish, J. & Norton, J.F. Sulfur effects on the internal carburization of Fe-Ni-Cr alloys. Oxid Met 26, 333–350 (1986). https://doi.org/10.1007/BF00659340
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00659340