Abstract
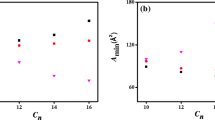
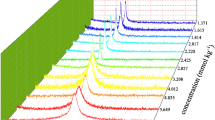
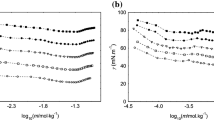
The micellar properties of a cationic fluorocarbon surfactant, diethanolheptadecafluoro-2-undecanolmethylammonium chloride (DEFUMAC), are studied by surface tension, fluorescence of pyrene-3-carboxaldehyde (PCA), and electric conductivity measurements. These measurements indicate that DEFUMAC solution provides a low surface tension and that its micelles present higher polar conditions under PCA than do cationic hydrocarbon surtactant micelles. Moreover, the micellar elongation induced by increasing concentrations of salt and surfactant concentration is observed by use of dynamic light scattering, transient electric birefringence, and viscosity measurements. The elongation by addition of salt can be explained by a multiple equilibrium description, while the elongation by increasing surfactant concentration is rapid above a particular concentration, viz. second CMC. Consequently, DEFUMAC micelle elongates more rapidly than do corresponding cationic hydrocarbon surfactants with chloride counterion.
Similar content being viewed by others
References
Shinoda K, Hato M, Hayashi T (1972) J Phys Chem 76:909–914
Hoffmann H, Kalus J, Thurn H (1983) Colloid Polym Sci 261:1043–1049
Holmes MC, Reynolds DJ, Boden N (1987) J Phys Chem 91:5257–5262
Angel M, Hoffmann H, Krämer U, Thurn H (1989) Ber Bunsenges Phys Chem 93:184–191
Berr SS, Jones RRM (1989) J Phys Chem 93:2555–2558
Johnson I, Olofsson G (1988) J Chem Soc, Faraday Trans 1 84:551–560
Forte G, Poggi Y, Appel J, Maret G (1984) J Phys Chem 88:5713–5720
Imae T, Abe A, Ikeda S (1988) J Phys Chem 92:1548–1553
Okuda H, Ozeki S, Ikeda S (1984) Bull Chem Soc Jpn 57:1321–1327
Tanaka A, Ikeda S (1991) Colloids and Surfaces 56:217–228
Kohler H-H, Strnad J (1990) J Phys Chem 94:7628–7634
Israelachvili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc, Faraday Trans 2 72:1525–1568
Missel PJ, Maszer NA, Benedek GB, Young CY (1980) J Phys Chem 84:1044–1057
Porte G, Appell J (1981) J Phys Chem 85:2511–2519
Ikeda S (1991) Colloid Polym Sci 269:49–61
Schorr W, Hoffmann H (1985) In: Degiorgio V, Corti M (eds) Physics of Amphiphiles: Micelles, Vesicles and Microemulsions. North-Holland Physics Publishing pp 160–180
Hoffmann H, Krämer U, Thurn H (1990) J Phys Chem 94:2027–2033
Stinger D (1966) J Phys Chem 70:1323–1325
Tanford C, Nozaki Y, Rohde MN (1977) J Phys Chem 81:1555–1560
Gamboa C, Sepúlveda L (1986) J Colloid Interface Sci 113:566–576
Funasaki N, Hada S, Neya S (1984) J Phys Chem 88:1243–1248
Kalyanasundaram K, Thomas JK (1977) J Phys Chem 81:2176–2180
Tamori K, Esumi K, Meguro K (1991) J Colloid Interface Sci 142:236–243
Evans HC (1956) J Chem Soc 579–586
Hoffmann H (1984) Ber Bunsenges Phys Chem 88:1078–1093
Mazer NA, Benedek GB, Carey MC (1976) J Phys Chem 80:1075–1085
Imae T (1988) J Phys Chem 92:5721–5726
Corti M, degiorgio V (1980) In: Light Scattering in Liquids and Macromolecular Solutions. Plenum Press pp 111–124
Atkins PW (1990) In: Physical Chemistry 4th ed. Oxford University Press pp 961
Broersma S (1960) J Chem Phys 32:1632–1635
Candau SJ, Hirsch E, Zana R, Adam M (1988) J Colloid Interface Sci 122:430–440
Imae T, Hashimoto K, Ikeda S (1990) Colloid Polym Sci 268:460–468
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tamori, K., Kihara, K., Esumi, K. et al. Micellar properties and growth of cationic fluorocarbon surfactant; diethanolheptadecafluoro-2-undecanolmethylammonium chloride. Colloid Polym Sci 270, 927–934 (1992). https://doi.org/10.1007/BF00657738
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00657738