Abstract
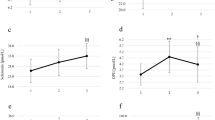
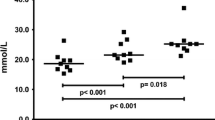
The effect of prolonged heavy physical exercise on serum galactosylhydroxylysyl glucosyltransferase activity (S-GGT) and serum type III procollagen aminoterminal propeptide (S-Pro(III)-N-P) concentration was studied in healthy male long-distance runners. S-GGT increased gradually by about 70% (p<0.01) during a competitive 24-h run, and a rising trend was also observed in S-Pro(III)-N-P. After the termination of the run S-GGT was normalized in two days, but the increase in S-Pro(III)-N-P continued up to one day after the race, reaching nearly 40% (p<0.005). The alterations in S-GGT and S-Pro(III)-N-P showed no significant correlation with S-CK, S-LDH or the distance run. The most likely explanation for the increases in S-GGT and S-Pro(III)-N-P is that prolonged heavy exercise injures the collagen-synthesizing cells of the connective tissue, leading to a short-term increase in type III procollagen production.
Similar content being viewed by others
References
Anttinen H (1977) Collagen glucosyltransferase activity in human serum. Clin Chim Acta 77:323–330
Anttinen H, Järvensivu PM, Savolainen E-R (1981) Serum galactosylhydroxylysyl glucosyltransferase in acute myocardial infarction and during subsequent collagen scar formation. Eur J Clin Invest 11:375–379
Bolarin D, Savolainen E-R, Kivirikko KI (1982) Enzymes of collagen synthesis and type III procollagen amino-propeptide in serum from Nigerians with hepatocellular carcinoma and other malignant diseases Int J Cancer 29:401–405
Bolarin D, Savolainen E-R, Kivirikko KI (1984) Three serum markers of collagen biosynthesis in Nigerians with eirrhosis and various infections disease. Eur J Clin Invest 14:90–95
Greenberg I, Arneson L (1967) Exertional rhabdomyolysis with myoglobinemia in a large group of military trainees Neurology 17:216–222
Higman B, Altland PD (1963) Effects of exercise and training on serum enzyme and tissue changes in rats. Am J Physiol 38:162–166
Kivirikko KI, Kuivaniemi H (1986) Post-translational modifications of collagen and their alterations in heritable diseases. In: Uitto J, Perejda AJ (eds) Diseases of connective tissue. The molecular pathology of the extracellular matrix. Marcel Dekker, New York, pp 263–292
Kivirikko KI, Myllylä R (1979) Collagen glycosyltransferases. Int Rev Conn Tissue Res 8:23–72
Kivirikko KI, Myllylä R (1982) Post-translational modifications. In: Weiss JB, Jayson MIV (eds) Collagen in health and disease. Churchill Livingstone, Edinburgh, pp 101–120
Kuutti-Savolainen E-R (1979) Enzymes of collagen biosynthesis in skin and serum in dermatological diseases. II. Serum enzymes. Clin Chim Acta 96:53–58
Kuutti-Savolainen E-R, Risteli J, Miettinen TA, Kivirikko KI (1979b) Collagen biosynthesis enzymes in serum and hepatic tissue in liver disease. I. Prolyl hydroxylase. Eur J Clin Invest 9:89–95
Kuutti-Savolainen E-R, Anttinen H, Miettinen TA Kivirikko KI (1979a) Collagen biosynthesis enzymes in serum and hepatic tissue in liver disease. II. Galactosylhydroxylysyl glucosyltransferase. Eur J Clin Invest 9:97–101
Mayne R (1982) Muscle. In: Weiss JB, Jayson MIV (eds) Collagen in health and disease. Churchill Livingstone, Edinburgh, pp 445–455
Menashi S, Grant ME (1979) Studies on the collagen glucosyltransferase activity present in platelets and plasma. Biochem J 178:777–784
Myllylä R, Risteli L, Kivirikko KI (1975) Assay of collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick embryo cartilage. Eur J Biochem 52:401–410
Myllylä R, Risteli L, Kivirikko KI (1976) Collagen glucosyltransferase. Partial purification and characterization of the enzyme from whole chick embryos and chick embryo cartilage. Eur J Biochem 61:59–67
Myllylä R, Myllylä VV, Tolonen U, Kivirikko KI (1982) Changes in collagen metabolism in diseased muscle. I. Biochemical studies. Arch Neurol 39:752–755
Myllylä R, Salminen A, Peltonen L, Takala TES, Vihko V (1986) Collagen metabolism of mouse skeletal muscle during the repair of exercise injuries. Pflügers Arch 407:64–70
Niemelä K, Palatsi I, Ikäheimo M, Takkunen J, Vuori J (1984) Evidence of impaired left ventricular performance after an uninterrupted competitive 24-hour run. Circulation 70:350–356
Peltonen L, Myllylä R, Tolonen U, Myllylä VV (1982) Changes in collagen metabolism in diseased muscle. II. Immunohistochemical studies. Arch Neurol 39:756–759
Prockop DJ, Kivirikko KI, Tuderman L, Guzman N (1979) The biosynthesis of collagen and its disorders. N Engl J Med 301:13–23, 77–85
Risteli L, Risteli J (1986) Radioimmunoassays for monitoring connective tissue metabolism. Rheumatology 10:216–245
Rojkind M (1984) The blue glass and predictive value of serum aminoterminal propeptide of type III procollagen as a marker of liver fibrosis. Hepatology 4:977–978
Rohde H, Vargas L, Hahn E, Kalbfleisch H, Bruguera M, Timpl R (1979) Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest 9:451–459
Rohde H, Langer I, Krieg T, Timpl R (1983) Serum and urine analysis of the aminoterminal procollagen peptide type III by radioimmunoassay with antibody Fab fragments. Coll Rel Res 3:371–379
Savolainen E-R, Goldberg B, Leo MA, Velez M, Lieber CS (1984) Diagnostic value of serum procollagen peptide measurements in alcoholic liver disease. Alcoholism: clinical and experimental research 8:384–389
Savolainen E-R, Micttinen TA, Pikkarainen P, Salaspuro M, Kivirikko KI (1983) Enzymes of collagen synthesis and type III procollagen amino-propeptide in the evaluation ofd-penicillamine and medroxyprogesterone treatments of primary biliary eirrhosis. Gut 24:136–142
Takala TES, Myllylä R, Salminen A, Anttinen H, Vihko V (1983) Increased activities of prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase enzymes of collagen biosynthesis, in skeletal muscle of endurance trained mice. Pflügers Arch 399:271–274
Vihko V, Salminen A, Rantamäki J (1978) Acid hydrolase activity in red and white skeletal muscle of mice during a two-week period following exhausting exercise. Pflügers Arch 378:99–106
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takala, T.E.S., Vuori, J., Anttinen, H. et al. Prolonged exercise causes an increase in the activity of galactosylhydroxylysyl glucosyltransferase and in the concentration of type III procollagen aminopropeptide in human serum. Pflugers Arch. 407, 500–503 (1986). https://doi.org/10.1007/BF00657507
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00657507