Abstract
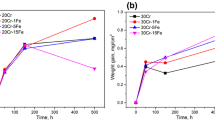
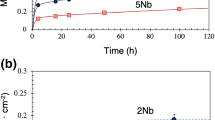
Structural Fe-Cr-Ni alloys rely upon a thermally formed, protective, surface oxide scale to prevent rapid corrosive degradation. The protective capacity of the surface scale may be strongly influenced by the alloy composition, with minor additions of reactive elements playing an important role in the initial stages of scale formation. The influence of an addition of Zr on initial scale growth on an Fe-Cr-Ni alloy has been investigated in situ utilizing an environmental cell incorporated into a high-voltage electron microscope. Oxidation experiments were conducted on a pure ternary Fe-Cr-Ni alloy and one containing 6 wt.% Zr for durations up to 1800 s. At 500°C in a low oxygen-partialpressure environment, a continuous surface oxide layer formed more quickly on the Zr-free alloy than on the Zr-modified alloy. Also, on the Zr-modified alloy, the scale was richer in Cr, and the rate of increase in oxide grain size was also greater.
Similar content being viewed by others
References
D. J. Baxter and K. Natesan,Proceedings of the Symposium on Corrosion Fossil Fuel Systems, Detroit, I. G. Wright, ed. (Electrochemical Society, 1983), pp. 213–232.
J. T. Prater and D. R. Baer, in Ref. 1, pp. 202–212.
S. J. Allan and M. J. Dean, Proceedings of the Petten International Conference on Behavior of High-Temperature Alloys in Aggressive Environments (Petten, The Netherlands (1979), pp. 319–334.
P. Moulin, F. Armanet, G. Béranger, and P. Lacome,Mémoires Scientifiques de la Revue de Métallurgie (1977), pp. 143–160.
G. C. Wood and F. H. Stott,Proceedings of the Conference on High-Temperature Corrosion,San Diego, R. A. Rapp, ed. (National Association of Corrosion Engineers, 1983), pp. 227–250.
D. J. Baxter and K. Natesan,Rev. High-Temp. Mat. 5, 149 (1983).
D. Bruce and P. Hancock,J. Inst. Met. 97, 140 (1969).
H. M. Flower and B. A. Wilcox,Corr. Sci. 17, 253 (1977).
H. M. Flower,J. Microscopy 97, 171 (1972).
R. K. DiCerbo and A. U. Seybolt,J. Am. Ceramics Soc. 42, 430 (1959).
D. Mortimer and M. L. Post,Corr. Sci. 8, 499 (1968).
K. A. Hay, F. G. Hicks, and D. R. Holmes,Werkstoffe Korr. 21, 917 (1970).
D. J. Baxter, R. C. Hurst, and R. T. Derricott,Werkstoffe Korr. 34, 446 (1983).
J. M. Perrow, W. W. Smeltzer, and J. D. Embury,Acta Met. 16, 1209 (1968).
A. Atkinson, R. I. Taylor, and A. E. Hughes,Philos. Mag. 45, 823 (1982).
T. T. Huang, B. Peterson, D. A. Shores, and E. Pfender,Corr. Sci. 24, 167 (1984).
D. J. Baxter and K. Natesan,Proceedings of the Symposium on High-Temperature Corrosion in Energy Systems, M. F. Rothman, ed. (The Metallurgical Society of AIME, 1985), pp. 237–252.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baxter, D.J., Natesan, K. A study of the initial stages of oxidation of Fe-Cr-Ni-Zr alloysin situ using a high-voltage electron microscope. Oxid Met 24, 331–350 (1985). https://doi.org/10.1007/BF00657064
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00657064