Abstract
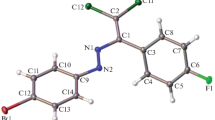
Crystal structures are reported for the molecular complexes ofE,E-1-[p-dimethylaminophenyl]-5-[o-hydroxyphenyl]-penta-1,4-dien-3-one (DHDK) with chloroform,m-dinitrobenzene andp-dimethylaminobenzaldehyde. The three complexes (first reported by I. M. Heilbron and J. S. Buck [1] in 1921) have different structures. In DHDK·0.4 CHCl3 (triclinic,a=12.086(6),b=10.323(5),c=8.015(4) Å, α=94.58(6), β=103.58(6), γ=110.10(6)o,Z=2,P \(\bar 1\) (assumed)), the host molecules are linked by two hydroxyl carbonyl hydrogen bonds to form centrosymmetric pairs, with the disordered CHCl3 molecules contained in cavities left between the molecule pairs. The complex is a clathrate. In DHDK·m-dinitrobenzene (triclinic,a=21.787(9),b-13.850(5),c=7.759(4) Å, α=88.25(5), β=84.70(5), γ=88.86(5)o,P \(\bar 1\),Z=4) the DHDK molecules are linked in ribbons through head-to-waist hydroxyl-carbonyl hydrogen bonds. The guest molecules are contained in sinuous channels left between the DHDK ribbons; the host and guest molecules are approximately coplanar. Successive planes are mutually shifted so that the guest molecules are enclosed above and below by host molecules. This is a new structural type, with features resembling those of channel inclusion complexes. In DHDK·p-dimethylaminobenzaldehyde (monoclinic,a=22.331(9),b=12.238(5),c=8.904(4) Å, β=92.99(5)o,Z=4,P21/n) the host molecules are arranged so as to leave channels of approximately rectangular cross-sections in which the guest molecules are accommodated. Additional stabilization is achieved by hydrogen bonding between host hydroxyl and guest carbonyl groups. This is a channel-inclusion complex. In the chloroform andp-dimethylaminobenzaldehyde complexes the host molecule has thes-trans, trans conformation but in them-dinitrobenzene complex its conformation iss-cis, trans.
Similar content being viewed by others
References
I. M. Heilbron and J. S. Buck:J. Chem. Soc. 119, 1500 (1921).
V. Balashov and H. D. Ursell:Acta Crystallogr. 10, 582 (1957).
L. V. Azaroff and M. J. Buerger:The Powder Method in X-ray Crystallography, Chapters 11 and 12. McGraw-Hill (1958).
S. L. Lawton: ‘A Cell Reduction Program’. Northwestern University (1967).
P. Main, M. M. Woolfson, L. Lessinger, G. Germain, and J. P. Declerq: MULTAN 77. A system of computer programmes for the automatic solution of crystal structures from X-ray diffraction data. Universities of York, England and Louvain, Belgium (1977).
M. R. Caira, R. G. F. Giles, L. R. Nassimbeni, G. M. Sheldrick and R. G. Hazell:Acta Crystallogr. B32, 1467 (1976).
G. M. Sheldrick: SHELX-77. A programme for crystal structure determination. University of Cambridge (1977).
W. C. Hamilton:Acta Crystallogr. 18, 502 (1965)
W. C. Hamilton:International Tables for X-ray Crystallography, Vol. IV, p. 288. The Kynoch Press (1974).
C. G. Pierpont and M. C. Mazza:Inorg. Chem 13, 1891 (1974).
T. Ukai, H. Kawazura, Y. Ishii, J. J. Bonnet, and J. A. Ibers:J. Organomet. Chem. 65, 253 (1974).
M. Laing:J. Chem. Soc. Perkin Trans. II, 1248 (1977).
F. H. Herbstein and W. Schwotzer:J. Am. Chem. Soc., in press (1984).
J. Preuss and A. Gieren:Acta Crystallogr. B31, 1276 (1975).
E. Dijkstra, A. T. Hutton, H. M. N. H. Irving, and L. R. Nassimbeni:Acta Crystallogr. B38, 535 (1982).
J. Bordner and P. Mullins:Crystallogr. Struct. Commun. 3, 693 (1974).
M. Grynpas and P. F. Lindley:Acta Crystallogr. B31, 2663 (1975).
F. H. Herbstein, W. Schwotzer, I. Addae-Mensah, F. G. Torto, and K. A. Woode:Acta Crystallogr. B37, 702 (1981).
J. Trotter and C. S. Williston:Acta Crystallogr. 21, 285 (1966).
A. Domenicano, A. Vaciago, and C. A. Coulson:Acta Crystallogr. B 31 221 (1975).
J. Reffner and W. C. McCrone:Anal. Chem. 31, 1119 (1959).
F. Iwasaki:Acta Crystallogr. B33, 1646 (1977).
F. van Bolhuis and C. Th. Kiers:Acta Crystallogr. B34, 1015 (1978).
I. Ikemoto, G. Katagiri, S. Nishimura, K. Yakushi, and H. Kuroda:Acta Crystallogr. B35, 2264 (1979).
Y. Ohashi, H. Iwasaki, and Y. Saito:Bull. Chem. Soc. J. 40, 1789 (1967).
F. H. Herbstein and M. Kaftory:Z. Kristallogr. 157, 1 (1981).
C. K. Johnson: ORTEP. Report ORNL-3794. Oak Ridge National Laboratory, Tennessee (1965).
L. Fallon III:Acta Crystallogr. B29, 2549 (1973).
G. S. Mandel and R. E. Marsh:Acta Crystallogr. B31, 2862 (1975).
F. H. Herbstein and M. Kapon:Phil. Trans. Roy. Soc., Lond. A291, 199 (1979).
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Friedrich Cramer on the occasion of his 60th birthday
Supplementary Data relating to this article are deposited with the British Library as Supplementary Publication NO. SUP 90076 (13 pages). To obtain copies, see page ii of this issue.
‘Molecular Compounds and Complexes’, Part XIV. For Part XIII, see [25].
Rights and permissions
About this article
Cite this article
Herbstein, F.H., Kapon, M., Reisner, G.M. et al. Crystallographic studies of the molecular complexes ofE,E-1[p-dimethylaminophenyl]-5-[o-hydroxyphenyl]-penta-1,4-dien-3-one 9DHDK) with chloroform (1:0.4),m-dinitrobenzene (1:1) andp-dimethylaminobenzaldehyde (1:1); the Heilbron complexes. Journal of Inclusion Phenomena 1, 233–250 (1984). https://doi.org/10.1007/BF00656759
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00656759