Abstract
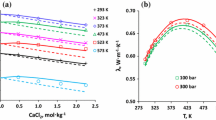
The specific heat capacities of the aqueous multicomponent system NaCl +KCl+MgCl2+CaCl2 with ionic strength between 8.3 and 9.6 (resembling Dead Sea waters) were measured between 15°C and 45°C. The obtained data were fitted to an empirical equation as a function of concentration and temperature. The thermodynamic functions of the studied multicomponent system were found to be strongly influenced by changes in MgCl2 concentrations. The application of Young's rule to such concentrated systems was checked at 25°C. The calculated (by Young's rule) specific heat capacitiesC p and apparent molar heat capacities Cp,ϕ of these multicomponent electrolyte solutions were in reasonable agreement with the measured values (−0.008 J-g−1-K−1 and −2.6 J-mol−1-K−1, respectively).
Similar content being viewed by others
References
B. S. Krumgalz and F. J. Millero,Mar. Chem. 11, 209 (1982).
F. J. Millero, G. Perron and J. E. Desnoyers,J. Geophys. Res. 78, 4499 (1973).
T. F. Young and M. B. Smith,J. Phys. Chem. 58, 716 (1954).
B. S. Krumgalz and F. Gat,Natl. Inst. Oceanogr., Haifa, Israel, Rep. H4/84 (1984).
D. Neev and K. O. Emery,Geol. Survey Israeli Bull. 41, (1967).
B. S. Krumgalz and F. J. Millero,Mar. Chem. 11, 477 (1982).
B. S. Krumgalz and R. Holzer,Limnol. Oceanogr. 25, 367 (1980).
P. Picker, E. Tremblay and C. Jolicoeur,J. Solution Chem. 3, 377 (1974).
G. Perron, J. E. Desnoyers and F. J. Millero,Can. J. Chem. 52, 3738 (1974).
P. Picker, P.-A. Leduc, P. R. Philip and J. E. Desnoyers,J. Chem. Thermodyn. 3, 631 (1971).
G. Perron, J.-L. Fortier and J. E. Desnoyers,J. Chem. Thermodyn. 7, 1177 (1975).
J.-L. Fortier and G. C. Benson,J. Chem. Thermodyn. 8, 411 (1976).
J. E. Desnoyers, C. de Visser, G. Perron and P. Picker,J. Solution Chem. 5, 605 (1976).
I. V. Olofsson,J. Chem. Thermodyn. 11, 1005 (1979).
G. S. Kell,J. Chem. Eng. Data 12, 66 (1967).
H. F. Stimson,Am. J. Phys. 23, 614 (1955).
G. Perron, A. Roux and J. E. Desnoyers,Can. J. Chem. 59, 3049 (1981).
J. E. Tanner and F. W. Lamb,J. Solution Chem. 7, 303 (1978).
Y. Marcus, inIonic Liquids, D. Inman and D. G. Lovering, eds., (Plenum, New York, 1981), p. 97.
K. S. Pitzer, inActivity Coefficients In Electrolyte Solution K. S. Pitzer, ed., (CRC Press, Boca Raton, 1991), p. 75.
J.-L. Fortier, P. A. Leduc and J. E. Desnoyers,J. Solution Chem. 3, 323 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krumgalz, B.S., Malester, I.A., Ostrich, I.J. et al. Heat capacities of concentrated multicomponent aqueous electrolyte solutions at various temperatures. J Solution Chem 21, 635–649 (1992). https://doi.org/10.1007/BF00650758
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00650758