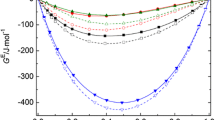
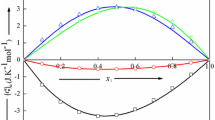
Abstract
The Flory theory of solution thermodynamics is used to predict exess volumes for systems containing a series of n-alkanes mixed with liquids of higher P* parameter and internal pressure, i.e., cyclopentane, cyclohexane, carbon tetrachloride, benzene and dioxane as well as of lower P*, i.e., decamethyltetrasiloxane. Trends of VE, e.g. changes of sign with alkane carbon number are well predicted and indicate the importance of the P* contribution in VE. Mixtures of hexane isomers with liquids of much higher P* typically have large excess enthalpies through zero as an S-shaped curve against composition which is negative on the side of the high P* component. This behaviour is interpreted as arising from increasingly large negative P* contributions in VE.
Similar content being viewed by others
References
H. T. Van and D. Patterson,J. Solution Chem. 11, 000 (1982).
I. Prigogine (with the collaboration of V. Mathot and A. Bellemans),The Molecular Theory of Solutions (North-Holland, Amsterdam, 1957; P. J. Flory,J. Am. Chem. Soc. 87, 1833 (1965).
I. Prigogine and V. Mathot,J. Chem. Phys. 20, 49 (1952).
E. Dickinson and I. A. McLure,J.C.S. Faraday I 70, 2328 (1974).
M. Barbe and D. Patterson,J. Solution Chem. 9, 753 (1980).
Y. P. Handa, C. M. Knobler, and R. L. Scott,J. Chem. Thermodyn.,9, 451 (1977).
D. Patterson,Pure and Appl. Chem. 57, 305 (1976).
M. A. Leiva, J. P. Greenberg, and C. M. Knobler,J. Chem. Eng. Data 24, 208 (1979).
R. S. Chahal, W. P. Kao, and D. Patterson,J.C.S. Faraday I 69, 1834 (1973).
J. R. Goates, J. B. Ott, and R. B. Grigg,J. Chem. Thermodyn,11, 497 (1979).
M. L. Martin and L. Symons,J. Chem. Thermodyn. 13, 81 (1981).
D. V. Fenby, J. R. Khurma, Z. S. Kooner, T. E. Block, C. M. Knobler, J. Reeder, and R. L. Scott,Aust. J. Chem 33, 1927 (1980).
V. T. Lam, P. Picker, D. Patterson, and P. Tancrede,J.C.S. Faraday II 70, 1465 (1974).
R. A. Orwell and P. J. Flory,J. Am. Chem. Soc. 89, 6814 (1967).
P. St. Romain, PhD. Thesis, McGill University (1977).
J. A. Riddick and W. B. Bunyer,Organic Solvents. Physical Properties and Methods of Purification, 3rd Ed., (Wiley Interscience, 1970).
R. R. Dreisbach,Physical Properties of Chemical Compounds, Vol II, (A.C.S. Washington, D. C. 1959).
B. P. Sahli, H. Gayer, and A. J. Richard,J. Chem. Thermodyn. 8, 179 (1976).
D. Allen, G. Gee, D. Mangaraj, D. Sims, and G. J. Wilson,Polymer 1, 456 (1960).
D. E. G. Jones, I. A. Weeks, and G. C. Benson,Can. J. Chem. 49, 2481 (1971).
G. C. Benson, S. Murakami, V. T. Lam, and J. SinghCan. J. Chem. 48, 211 (1970).
A. Abe and P. J. Flory,J. Am. Chem. Soc. 87, 1838 (1965).
V. H. Khan and S. V. Subrahhmanyan,J.C.S. Faraday I 67, 2282 (1971).
A. C. Gupta and R. W. HanksThermochimica Acta 21, 143 (1977).
P. Tancrede, D. Patterson, and V. T. Lam,J.C.S. Faraday II 71, 985 (1975).
H. T. Van, This laboratory, Unpublished data.
A. Inglese and J.P.E Grolier, Int. Data Series, Selected Data on Mixtures Ser A, 1975, pp. 98–99.
M. Barbe, PhD. Thesis, McGill University, 1978.
J. M. Berryman and E. L. Heric,Can. J. Chem. 50, 3799 (1972).
A. Inglese and J. P.E. Grolier, Int. Data Series, Selected Data on Mixtures Ser A, 1975, Ref. 27, p. 1.
A. Inglese and J. P.E. Grolier, Int. Data Series, Selected Data on Mixtures Ser A, 1975, Ref. 27, p. 74.
B. S. Harsted and E. S. Thomsen,J. Chem. Thermodyn. 6, 557 (1974).
L. Romani and M. I. Paz-Andrade, Int. Data Series, Selected Data on Mixtures, Ser. A, 1973, p. 100.
M. D. Guillen and C. Gutierrez Losa,J. Chem. Thermodyn. 10, 567 (1978).
A. Inglese, E. Wilhelm, J. P. E. Grolier, and H. V. Kehiaian,J. Chem. Thermodyn. 12, 217 (1980).
Y. P. Handa, C. M. Knobler, and R. L. Scott,J. Chem. Thermodyn. 9, 451, (1977).
W. L. Spiteri and T. M. Letcher,J. Chem. Thermodyn. 11, 667 (1979).
This laboratory, V. Dohnal.
M. A. Siddiqi, G. Götze, and F. Kohler,Ber. Bunsenges Phys. Chem. 84, 529 (1980).
B. S. Mahl, R. K. Nigam, S. L. Chopra and P. P. Singh,J. Chem. Thermodyn. 3, 363 (1971).
T. M. Letcher,J. Chem. Thermodyn. 7, 205 (1975).
T. M. Letcher and W. L. Spiteri,J. Chem. Thermodyn. 11, 435 (1979).
T. G. Bissel, G. E. Okafor, and A. G. Williamson,J. Chem. Thermodyn. 3, 393 (1971).
D. V. S. Jain, B. S. Lark, S. S. Chamak, and P. Chandler,Ind. J. Chem. 8, 66 (1970).
M. Diaz Peña and J. N. Delgado,Ann. Quim. 70, 678 (1974).
A. W. Andrews and K. W. Morcom,J. Chem. Thermodyn. 3, 513 (1971).
A. Inglese, J. P. E. Grolier, and E. Wilheim,J. Chem. Eng. Data, in press.
This laboratory, D. Nikolic.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costas, M., Patterson, D. Volumes of mixing and theP * effect: Part II. Mixtures of alkanes with liquids of different internal pressures. J Solution Chem 11, 807–821 (1982). https://doi.org/10.1007/BF00650520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00650520