Abstract
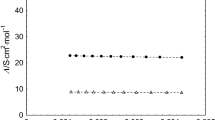
Conductance data are reported for Ph4AsPic, Ph4PPic, Ph4SbPic, Hex4NPic, Bu4PPic, Et4NSbCl6 in propylene carbonate at 25°C in the concentration range 1×10−4 to 15×10−4 M. The data were analyzed by the Justice modification of the Fuoss-Hsia equation and all salts studied were found to be associated and to form solvent separated ion pairs. Application of the Barthel-Bjerrum model of ion association permitted calculation of the non coulombic portion of the potentials of mean force, W±. Ionic limiting equivalent conductances of six ions were calculated using known values of R4N+, and Pic− ions. Walden products of ions in propylene carbonate were examined in the light of modern ion mobility theories, including Boyd-Zwanzig, Hubbard-Onsager, and Hubbard-Kayser models of ion solvent interactions.
Similar content being viewed by others
References
J. Barthel, H. J. Gores, G. Schmeer, and R. Wachter,Topics in Current Chemistry, Vol. III (Springer Verlag, Heidelberg, 1982), p. 114.
H. V. Venkatasetty,Lithium Battery Technology (The Electrochemical Society, Pennington, New Jersey, 1984), Chap. 1.
R. Jasinski, inAdvances in Electrochemistry and Electrochemical Engineering, Vol. 8, C. W. Tobias, ed. (Wiley, New York, 1971).
W. H. Lee, inChemistry of Nonaqueous Solvents, Vol. 4, J. J. Lagowski, ed. (Academic Press, New York, 1976), Chap. 6.
J. F. Reardon,Electrochim. Acta 32, 1595 (1987).
N. N. Lichtin, B. Wasserman, E. Clougherty, J. Wasserman, and J. F. Reardon,J. Phys. Chem. 84, 2946 (1980).
G. W. Covell, A. Ledwigh, A. C. White, and H. J. Woods,J. Chem. Soc. (B) 227, (1970).
R. M. Fuoss and K. L. Hsia,Proc. Ntl. Acad. Sci., USA 57, 1550 (1967).
R. Fernandez-Prini, inPhysical Chemistry of Organic Solvent Systems A. K. Covington and J. Dickenson, eds. (Plenum Press, New York, 1973), p. 565.
J. C. Justice,J. Chim. Phys. 65, 353, (1968).
R. Fernandez-Prini,Trans. Faraday Soc. 64, 2146 (1968).
T. Shedlovsky,J. Franklin Inst. 225, 739 (1938).
N. N. Lichtin, B. Wasserman, and J. F. Reardon,J. Phys. Chem. 85, 1590 (1981).
M. Salomon and E. J. Plichta,Electrochim. Acta 28, 1681 (1983).
R. L. Kay,J. Am. Chem. Soc. 82, 2099 (1960).
R. Zana, J. E. Desnoyers, G. Parron, R. L. Kay, and K. Lee,J. Phys. Chem. 86, 3996 (1982).
R. L. Kay,Water, A Comprehensive Treatise, Vol. 3, F. Frank, ed. (Plenum Press, New York, 1973), Vol. 3, Chap. 4.
J. Barthel, R. Wachter, and H. J. Gores,Modern Aspects of Electrochemistry, Vol. 13, B. E. Conway and J. O'M. Bockris, eds. (Plenum Press, New York, 1979), Chap. 1.
J. C. Coupez and M. L'Her,C. R. Acad. Sci. Ser C. 271, 357 (1970).
K. Izutsu, I. M. Kolthoff, T. Fujinaga, M. Hattori, and M. K. Chantooni,Anal. Chem. 49, 503 (1977).
A. F. Wells, inStructural Inorganic Chemistry, (Oxford University Press, 1975), p. 746.
M. Born,Z. Phys. 1, 221 (1920).
R. M. Fuoss,Proc. Ntl. Acad. Sci. 45, 807 (1959).
R. H. Boyd,J. Chem. Phys. 35, 1281 (1961),39, 2376 (1963).
R. Zwanzig,J. Chem. Phys. 52, 3625 (1970).
J. Barthel,Pure and Appl. Chem. 57, 355 (1985).
R. Fernandez-Prini and G. Atkinson,J. Phys. Chem. 75, 239 (1971).
J. B. Hubbard and L. Onsager,J. Chem. Phys. 67, 4850 (1977).
J. B. Hubbard,J. Chem. Phys. 68, 1649 (1978).
D. F. Evans, T. Tominaga, J. B. Hubbard, and P. G. Wolynes,J. Phys. Chem. 83, 2669 (1979).
J. H. Chen and S. A. Adelman,J. Chem. Phys. 72, 2819 (1980).
J. B. Hubbard and R. F. Kayser,J. Chem. Phys. 74, 3635 (1981).
P. J. Stiles,Chem. Phys. Lett. 80, 73 (1981).
J. B. Hubbard and R. F. Kayser,Chem. Phys. 66, 377 (1982).
P. J. Stiles, J. B. Hubbard, and R. F. Kayser,J. Chem. Phys. 77, 6189 (1982).
F. Booth,J. Chem. Phys. 19, 391 (1951).
L. Onsager,J. Am. Chem. Soc. 58, 1486 (1936).
F. Accasina, A. D'Aprano, and R. M. Fuoss,J. Am. Chem. Soc. 81, 1058 (1951).
H. L. Yeager, J. D. Fedyk, and R. J. Parker,J. Phys. Chem. 77, 2407 (1973).
H. Feng-Chun and W. R. Gilkerson,J. Solution Chem. 12, 161 (1983).
A. K. Covington and T. Dickinson, inPhysical Chem. of Organic Solvent Systems, A. K. Covington and T. Dickinson, eds. (Plenum Press, New York, 1973), Chap. 1.
H. Finegold,J. Phys. Chem. 72, 3244 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McDonagh, P.M., Reardon, J.F. II. Ionic association and mobility in propylene carbonate. J Solution Chem 19, 301–314 (1990). https://doi.org/10.1007/BF00650460
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00650460