Abstract
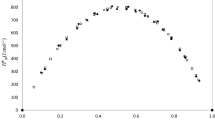
Molar excess volumes and partial molar volumes are reported for binary mixtures of 1,4-dioxane + acetonitrile, n-butylamine + acetonitrile and n-butylamine + 1,4-dioxane at five different temperatures and over the complete concentration range. The Prigogine-Flory-Patterson model of solution thermodynamics has been used to predict the excess molar volumes. This work shows the importance of the three contributions: interactional, internal pressure and free volume, to the excess volume.
Similar content being viewed by others
References
I. L. Acevedo, M. A. Postigo, and M. Katz,J. Solution Chem. 17, 977 (1988).
M. L. G. de Soria, J. L. Zurita, M. A. Postigo, and M. Katz,Thermochim. Acta 130, 249 (1988).
M. Sreenivasulu and P. R. Naidu,J. Chem. Thermodyn. 10, 731 (1987).
G. E. Papanastasiou, A. D. Papoutsis, and G. T. Kokkinidis,J. Chem. Eng. Data 32, 377 (1987).
K. Ramanjaneyulu, A. C. H. Chandrasekhar, P. Venkateswarlu, and A. Krishnaiah,Phys. Chem. Liq. 16, 217 (1987).
I. F. Pierola and A. Horta,J. Chim. Phys. 77, 271 (1980).
J. A. Riddick, N. B. Bunger, and T. Sakano,Organic Solvents, 4th edn., (Wiley, New York, 1986).
J. S. Sandhu, A. K. Sharma, and R. K. Wadi,J. Chem. Eng. Data 31, 152 (1986).
J. C. Schurz and W. M. Chang,J. Phys. Chem. 75, 938 (1971).
R. J. Fort and W. R. Moore,Trans. Faraday Soc. 62, 1112 (1966).
M. C. Prolongo, M. R. Masegosa, I. H. Fuentes, and A. Horta,J. Phys. Chem. 88, 2163 (1984).
I. Nagata and K. Tamura,Thermochim. Acta 124, 53 (1988).
E. P. Rao and K. S. Reddy,Fluid Phase Eq. 34, 265 (1987).
C. de Visser, W. J. M. Heuvesland, L. A. Dunn, and G. Somsen,J. Am. Chem. Soc. Faraday Trans. I 74, 1159 (1978).
Y. Syrkin and E. Schott-Lvova,Acta Physicochim., U.R.S.S. 19, 379 (1944).
H. T. Van and D. Patterson,J. Solution Chem. 11, 793 (1984).
I. Prigogine,The Molecular Theory of Solutions (North Holland, Amsterdam, 1957).
P. J. Flory,Disc. Faraday Soc. 49, 7 (1970).
P. Tancrede, P. Bothoerl, P. de St. Romain, and D. Patterson,J. Chem. Soc. Faraday Trans. II 73, 15 (1977).
P. de St. Romain, G. H. T. Van, and D. Patterson,J. Chem. Soc. Faraday Trans. II 75, 1700 (1979).
P. J. Flory, R. A. Orwoll, and A. Vrij,J. Am. Chem. Soc. 86, 3507 and 3515 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Schaefer, C.R., Davolip, F. & Katz, M. Molar excess volumes of 1,4-dioxane+ acetonitrile,n-butylamine+acetonitrile andn-butylamine+1,4-dioxane at different temperatures. J Solution Chem 19, 289–299 (1990). https://doi.org/10.1007/BF00650459
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00650459