Abstract
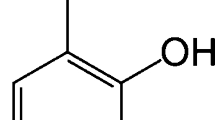
Experimental data are presented for the solubility in water of benzoic and toluic acids from 5° to 65°C. From the solubility the molality of the monomeric form of the acid is calculated using literature data for both ionization and dimerization of the acid. These data for the monomer combined with data from the literature for vaporization of the solid and ionization in both the gas phase and the aqueous phase yield entropy and enthalpy changes for the solvation of molecular and anionic forms of the acid. A similar procedure is also applied to literature data for the solubility of benzene in water. It is shown that the hydration entropies of the monomeric forms are a linear function of their partial molar volumes. It is concluded that hydration of the undissociated o-toluic acid may be crucial to the increased acidity of that acid compared to benzoic acid.
Similar content being viewed by others
References
L. E. Strong, D. J. Blubaugh, J. B. Kallmyer, S. P. Taylor,J. Phys. Chem. 89, 1318 (1985).
L. E. Strong, C. L. Brummel, P. Lindower,J. Solution Chem. 16 105 (1987).
E. M. Arnett, L. E. Small, D. Oaucea, D. Johnston,J. Am. Chem. Soc. 98, 7346 (1976).
E. M. Arnett,J., Chem. Educ. 62, 385 (1985).
T. B. McMahon and P. Kebarle,J. Am. Chem. Soc. 99, 2222 (1977).
M. Colomina, P. Jimenez, M. V. Roux, C. Turrion, Anales de Quimica,Series A 82, 126 (1986).
M. Colomina, P. Jimenez, and C. Turrion,J. Chem. Thermo. 14, 779 (1982).
L. E. Strong, C. L. Brummel, R. Ryther, J. R. Radford, and A. D. Pethybridge,J. Solution Chem. 12 1145 (1988).
B. B. Owen, R. C. Miller, C. E. Milner, H. L. Hogan,J. Phys. Chem. 65, 2065 (1961).
H. F. Halliwell and L. E. Strong,J. Phys. Chem. 89, 4137 (1985).
H. L. Ward and S. S. Cooper,J. Phys. Chem. 24, 1484 (1930).
D. S. Arnold, C. A. Plank, E. E. Erickson, and F. P. Pike,Ind. Eng. Chem. Chem. Eng. Data Ser.3, 253, (1958).
F. Franks, M. Gent, and H. H. Johnson,J. Chem. Soc. 1963, 2716, (1963).
S. J. Gill, N. F. Nichols, and I. Wadsö,J. Chem. Thermo. 8, 445, (1976).
E. E. Tucker, E. H. Lane, and S. D. Christian,J. Solution Chem. 10, 1 (1981).
F. W. Schwab and E. Wichers,J. Res, NBS,34, 333 (1945).
R. W. G. Wyckoff,Crystal Structures, Interscience Publishers, New York, 1969, vol 6, Part 1, p. 17. see also: G. A. Sims, J. M. Robertson, T. H. Goodwin,Acta Cryst. 8, 157 (1955).
C. G. de Kruif, and J. G. Blok,J. Chem. Thermo. 14, 201 (1982).
F. Franks and D. S. Reid,Water:A Comprehensive Treatise F. Franks (ed.), Plenum Press, New York (1973) vol 2, Chap. 5.
G. Perron and J. E. Desnoyers,Fluid Phase Equilibria 2, 239 (1979).
K. Abi-Saleh, personal communication.
S. D. Hamann and S. C. Lim,Austral. J. Chem. 7, 329 (1954).
G. H. Parsons, C. H. Rochester, and C. E. C. Wood,J. Chem. Soc. (B)1971, 533 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Strong, L.E., Neff, R.M. & Whitesel, I. Thermodynamics of dissolving and solvation processes for benzoic acid and the toluic acids in aqueous solution. J Solution Chem 18, 101–114 (1989). https://doi.org/10.1007/BF00649567
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00649567