Abstract
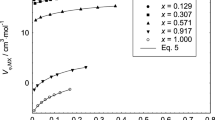
A flow densimeter and an ultrasonic sound velocimeter have been used to measure densities and isentropic compressibilities of solutions of LiBr, NaCl, NaBr, Nal, KF, KCl, KBr, Kl, RbBr, Rbl, CsF, CsBr, Ph 4 PBr, and NaBPh 4 in anhydrous methanol at 25°C. the latter two electrolytes were also investigated in water at 25°C. Concentrations ranged from about 0.005 m to above 0.25m, solubility permitting. Apparent molar isentropic compressibilities, KS,ϕ, have been calculated and extrapolated to infinite dilution to obtain K oS,ϕ . The K oS,ϕ values in methanol are all negative, and significantly more negative than the corresponding data in water. Additional data from the literature for acetonitrile and ethanol solutions show that K oS,ϕ for the alkali metal halides become more negative in direct proportion to increasing solvent isentropic compressibility. Furthermore, the dependence of K oS,ϕ in ionic size also varies in proportion to solvent isentropic compressibility. An explanation of this behavior is presented.
Similar content being viewed by others
References
R. Garnsey, R. J. Boe, R. Mahoney, and T. A. Litovitz,J. Chem. Phys. 50, 5222 (1969).
J. G. Mathieson and B. E. Conway,J.C.S. Faraday Trans. I 70, 752 (1974).
J. G. Mathieson and B. E. Conway,J. Solution Chem. 3, 455 (1974).
F. T. Gucker, D. Stubley, and D. J. Hill,J. Chem. Thermodyn. 7, 865 (1975).
M. Sakurai, T. Nakajima, T. Komatsu, and T. Nakagama,Chem. Letters (9), 971 (1975).
F. J. Millero, G. K. Ward, and P. V. Chetirkin,J. Acous. Soc. Amer. 61, 1492 (1977).
F. T. Gucker, D. Stubley, and D. J. Hill,J. Chem. Thermodyn. 9, 987 (1977).
E. Ayranci and B. E. Conway,J.C.S. Faraday Trans. 1 79, 1357 (1983).
F. J. Millero, J. Ricco, and D. R. Schreiber,J. Solution Chem. 11, 671 (1982).
H. Holland and O. J. Kvammen,J. Chem. Eng. Data 28, 179 (1983).
F. Kawaizumi, K. Matsumoto, and H. Nomura,J. Phys. Chem. 87, 3161 (1983).
I. Davidson, G. Perron, and J. E. Desnoyers,Can. J. Chem. 59, 2212 (1981).
A. S. Kaurova and G. P. Roshchina,Soviet Phys.-Acous. 12, 95 (1966).
A. S. Kaurova and G. P. Roshchina,Soviet Phys-Acous. 12, 276 (1967).
D. S. Allam, Ph.D. Thesis, University of London (1963).
D. S. Allam, and W. H. Lee,J. Chem. Soc. 6049 (1964).
D. S. Allam and W. H. Lee,J. Chem. Soc. (A)6, 5 (1966).
P. Picker, E. Tremblay, and C. Jolicoeur,J. Solution Chem. 3, 377 (1974).
A. J. Pasztor and C. M. Criss,J. Solution Chem. 7, 27 (1978).
C. Shin and C. M. Criss, unpublished data.
Instruction Manual for Nusonics Model 6105 Sonic Solution Monitor.
V. A. Del Grosso and C. W. Mader,J. Acoust. Soc. Amer. 52, 1442 (1972).
J. Lara and J. E. Desnoyers,J. Solution Chem. 10, 465 (1981).
O. Kiyohara and G. C. Benson,J. Solution Chem. 10, 281 (1981).
B. Pesce and A. Giacomini,Ric. Sci. 11, 619 (1940).
W. D. T. Dale, P. A. Flarelle, and P. Kruus,Can. J. Chem. 54, 355 (1976).
J. Emergy and S. Gasse,Acustica 43, 205 (1979).
R. C. Wilhoit and B. J. Swolinski,J. Phys. Chem. Ref. Data 2, 1 (1973).
D. O. Masson,Phil. Mag. 8, 218 (1929).
O. Redlich and P. Rosenfeld,Z. Electrochem. 37, 705 (1931); D. Redlich and D. M. Meyer,Chem. Rev. 64, 221 (1964).
F. J. Millero, inActivity Coefficients in Electrolyte Solutions, Vol. 2, R. M. Pytkowicz, ed., (CRC Press, Boca Raton, 1979).
F. J. Millero,J. Solution Chem. 2, 1 (1973).
P. S. Z. Rogers, and K. S. Pitzer,J. Phys. and Chem. Ref. Data 11, 15 (1982).
B. B. Owen, R. C. Miller, C. E. Milner, and H. L. Cogan,J. Phys. Chem. 65, 2065 (1961).
C. Chem, R. A. Fine, and F. J. Millero,J. Chem. Phys. 66, 2142 (1977).
H. Hartmann, A. Neumann, and G. Rinck,Z. Phys. Chem. Neue Folge 44, 204 (1965).
E. Schadow and R. Steiner,Z. Phys. Chem. Neue Folge,66, 105 (1969).
K. Srinivasan and R. L. Kay,J. Solution Chem. 4, 299 (1975);6, 357 (1977).
B. Sahli, H. Gager, and A. Richard,J. Chem. Thermodyn. 8, 179 (1976).
B. E. Conway and R. E. Verrall,J. Phys. Chem. 70, 3952 (1966).
L. H. Laliberte and B. E. Conway,J. Phys. Chem. 74, 4116 (1970).
F. Kawaizumi and R. Zana,J. Phys. Chem. 78, 627 (1974).
M. Dack,Austr. J. Chem. 29, 779 (1976).
D. Bradley and K. Pitzer,J. Phys., Chem. 12, 1599 (1979).
R. French and C. M. Criss,J. Solution Chem. 11, 625 (1982).
L. G. Hepler,J. Phys. Chem. 61, 1426 (1957).
Y. Choi and C. M. Criss,Disc. Faraday Soc. 64, 204 (1978).
R. Zana, G. A. Lage, and C. M. Criss,J. Solution Chem. 9, 667 (1980).
J. T. Edward,J. Chem. Educ. 47, 261 (1970).
C. M. Criss, R. P. Held, and E. Luksha,J. Phys. Chem. 74, 2970 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lankford, J.I., Holladay, W.T. & Criss, C.M. Isentropic compressibilities of univalent electrolytes in methanol at 25°C. J Solution Chem 13, 699–720 (1984). https://doi.org/10.1007/BF00649010
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00649010