Abstract
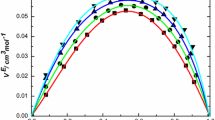
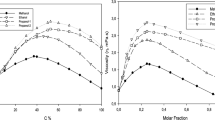
Viscosity measurements have been made at 25°C on solutions of water inn-propanol, and at 15, 25, 35, and 45°C on solutions of water inn-butanol,n-pentanol, andn-hexanol over the respective solubility ranges. For most of the systems, water decreases the viscosity of the dry alcohols, while for the lower members of the series literature data report an increase in viscosity on addition of water. These results are rationalized in terms of two kinds of interaction between water molecules and alcohols: participation of water molecules in chain formation for the lower alcohols and formation of water-centered complexes for butanol and higher alcohols.
Similar content being viewed by others
References
A. D'Aprano,J. Phys. Chem. 23, 1756 (1974).
A. D'Aprano, I. D. Donato, E. Caponetti, and V. Agrigento,Gazz. Chim. Ital. 108, 601 (1978).
A. D'Aprano and V. Agrigento,Gazz. Chim. Ital. 108, 703 (1978).
A. D'Aprano, I. D. Donato, and E. Caponetti,J. Solution Chem. 8, 135 (1979).
J. H. Hogan, R. E. Engel, and H. F. Stevenson,Anal. Chem. 42, 249 (1970).
H. T. Hall and R. M. Fuoss,J. Am. Chem. Soc. 73, 265 (1951).
M. Goffredi and T. Shedlovsky,J. Phys. Chem. 71, 2176 (1967).
J. F. Swindell, J. R. Cole, and T. B. Godfrey,J. Natl. Bur. Stand. 48, 1 (1952).
A. Than and E. S. Amis,Z. Phys. Chem. (Frankfurt) 58, 196 (1968).
R. L. Kay, B. J. Hales, and G. P. Cunningham,J. Phys. Chem. 71, 3925 (1967).
G. J. Janz and A. E. Marcinkowsky,Bull. Nat. Inst. Sci. India 29, 188 (1965).
J. Timmermans and F. Martin,J. Chim. Phys. 23, 733 (1926).
M. R. Cannon,Anal. Chem. 32, 355 (1960).
J. L. Hawes and R. L. Kay,J. Phys. Chem. 69, 2420 (1965).
L. G. Pedersen and E. S. Amis,Z. Phys. Chem. (Frankfurt) 36, 199 (1963).
T. Shedlovsky and R. L. Kay,J. Phys. Chem. 60, 151 (1956).
M. G. Foster and E. S. Amis,Z. Phys. Chem. Neue Folge 3, 365 (1955).
A. S. C. Lawrence, M. P. McDonald, and J. V. Stevens,Trans. Faraday Soc. 65, 323 (1969).
B. X. Lippold and M. S. Adel,Arch. Pharm. (Weinheim Ger.) 305, 418 (1979).
P. G. Sears, R. R. Holmes, and L. R. Dawson,J. Electrochem. Soc. 102, 145 (1955).
P. Walden,Z. Phys. Chem. 54, 129 (1906).
E. D. Copley, D. M. Murray-Rust, and H. Hartley,J. Chem. Soc., 2492 (1930).
G. J. Janz and S. S. Danyluk,Chem. Rev. 60, 209 (1960).
T. Titani,Bull. Inst. Res. Jpn., 671 (1927).
J. Timmermans and Y. Delcourt,J. Chim. Phys. 31, 85 (1934).
W. H. Zachariasen,J. Chem. Phys. 3, 158 (1935).
A. C. Brown and D. J. G. Ives,J. Chem. Soc., 1608 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
D'Aprano, A., Donato, I.D., Caponetti, E. et al. Viscosity studies of solutions of water inn-aliphatic alcohols at various temperatures. J Solution Chem 8, 793–800 (1979). https://doi.org/10.1007/BF00648578
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00648578