Abstract
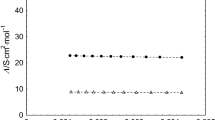
The cation- and anion-constituent transference numbers of aqueous 0.02M potassium picrate have been measured at 25°C by the moving-boundary method. The large difference (0.014) between the results and the predictions of the Debye-Hückel-Onsager theory, and similar anomalies in the literature, are consistent with the existence of picrate ion dimers (Pi 2−2 ) with a formation constant of 4(±2) liter-mole−1.
Similar content being viewed by others
References
D. J. G. Ives and P. G. N. Moseley,J. Chem. Soc. B, 757 (1966).
M. Shamim and M. Spiro,Trans. Faraday Soc. 66, 2863 (1970).
H. H. Broene and T. De Vries,J. Am. Chem. Soc. 69, 1644 (1947).
A. D. Pethybridge and J. E. Prue,Trans. Faraday Soc. 63, 2019 (1967).
M. Selvaratnam and M. Spiro,Trans. Faraday Soc. 61, 360 (1965).
J. Lange and E. Herre,Z. Physik. Chem. A 181, 329 (1938).
A. Seidell and W. F. Linke,Solubilities of Inorganic and Metal-Organic Compounds (A. C. S., Washington, D. C., 1965), 4th ed., Vol. II, p. 53.
M. Spiro, “Transference Numbers”, inPhysical Methods of Chemistry, Part II A: Electrochemical Methods, A. Weissberger and B. W. Rossiter, eds., (Interscience Publishers, New York, 1971), Chap. 4.
J. M. Notley and M. Spiro,J. Phys. Chem. 70, 1502 (1966).
L. G. Longsworth,J. Am. Chem. Soc. 54, 2741 (1932).
M. Ohag and P. G. N. Moseley, unpublished work.
Calculated from the data listed by F. J. Millero,Chem. Rev. 71, 147 (1971).
A. I. Vogel,A Textbook of Quantitative Inorganic Analysis (Longmans, London, 1961), 3rd ed., p. 444.
W. Jaekel,Z. Physik. Chem. (Leipzig),207, 296 (1957).
R. A. Robinson and R. H. Stokes,Electrolyte Solutions (Butterworths, London, 1965), 2nd ed. revised, (a) Appendix 6.1, (b) Appendix 7.1, (c) p. 144.
G. Kortüm and A. Weller,Z. Naturforsch. 5a, 451 (1950).
H. M. Daggett, Jr., E. J. Bair, and C. A. Kraus,J. Am. Chem. Soc. 73, 799 (1951); M. J. McDowell and C. A. Kraus,J. Am. Chem. Soc. 73, 2170 (1951).
G. Kortüm and H. Wilski,Z. Physik. Chem. (Frankfurt),2, 256 (1954).
R. H. Stokes,J. Am. Chem. Soc. 76, 1988 (1954).
R. L. Kay and J. L. Dye,Proc. Nat. Acad. Sci. U.S. 49, 5 (1963).
G. Kortüm and A. Weller,Z. Naturforsch. 5a, 590 (1950).
Author information
Authors and Affiliations
Additional information
This work was carried out at Imperial College while P. G. N. Moseley was on leave of absence from the University of Khartoum.
Rights and permissions
About this article
Cite this article
Moseley, P.G.N., Spiro, M. Transference numbers of potassium picrate in water at 25°C and the dimerization of picrate ions. J Solution Chem 1, 39–43 (1972). https://doi.org/10.1007/BF00648415
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00648415