Abstract
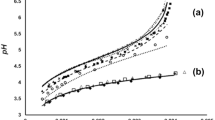
Values of pa oH for 0.05 mole-kg−1 aqueous solutions of sodium hydrogen diglycolate in the temperature range 5–65°C have been obtained from cells without transport, and can be fitted to the equation
The analysis has been carried out by a multilinear regression procedure using a form of the Clarke and Glew equation. This buffer standard may be a useful alternative to the saturated potassium hydrogen tartrate buffer.
Similar content being viewed by others
References
D. A. Keyworth and R. B. Hahn,Anal. Chem. 30, 1343 (1958).
D. A. Keyworth and R. B. Hahn,Talanta 1, 41 (1958).
Science Citation Index (Institute for Scientific Information, Philadelphia, Pa.).
R. G. Bates,Determination of pH. Theory and Practice, 2nd edn. (Wiley, New York, 1973), p. 283.
A. K. Covington and J. E. Prue,J. Chem. Soc., 3696 (1955).
H. P. Bütikofer and A. K. Covington,Anal. Chim. Acta 108, 179 (1979).
F. G. K. Baucke,Chem. Eng. Tech. 49, 739 (1977).
F. G. K. Baucke, private communication, Nov. 78.
R. G. Bates,Determination of pH. Theory and Practice, 2nd edn. (Wiley, New York), p. 50.
R. G. Bates and E. A. Guggenheim,Pure Appl. Chem. 1, 163 (1961).
W. J. Hamer and S. F. Acree,J. Res. Natl. Bur. Stand. 32, 215 (1944).
R. G. Bates,Determination of pH. Theory and Practice, 2nd edn. (Wiley, New York), p. 449.
R. G. Bates,Determination of pH. Theory and Practice, 2nd edn. (Wiley, New York), p. 72.
E. C. W. Clarke and D. N. Glew,Trans. Faraday Soc. 62, 539 (1966).
H. P. Bütikofer, A. K. Covington, and D. A. Evans,Electrochim. Acta 25, 1071 (1979).
A. K. Covington and M. J. Rebelo, to be published.
D. J. Alner, J. J. Greczek, and A. G. Smeeth,J. Chem. Soc. A. 1205 (1967).
R. G. Bates, V. E. Bower, R. G. Miller, and E. R. Smith,J. Res. Natl. Bur. Stand. 47, 433 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Covington, A.K., Cairns, J. Determination of standard pH values for sodium hydrogen diglycolate buffer (0.05m) from 5–65°C. J Solution Chem 9, 517–523 (1980). https://doi.org/10.1007/BF00647740
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00647740