Abstract
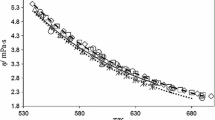
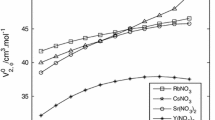
The density, viscosity, conductance, and transference number of aqueous magnesium chloride at 298.15°K are reported for the concentration range 0 to 5.7m. The transference numbers were determined using the emf method. It was found that the viscosity, conductance, and transference number of aqueous magnesium chloride are similar to those for aqueous nickel chloride. It was therefore tentatively concluded that incomplete dissociation and complex formation was unlikely to be of importance in nickel chloride solutions.
Similar content being viewed by others
References
H. G. Hertz,Ber. Bunsenges. Phys. Chem. 81, 656 (1977).
H. G. Hertz, K. R. Harris, R. Mills and L. A. Woolf,Ber. Bunsenges. Phys. Chem. 81, 664 (1977).
H. G. Hertz and R. Mills,J. Phys. Chem. 82, 952 (1978).
R. H. Stokes, S. Phang, and R. Mills,J. Solution Chem. 8, 489 (1979).
S. Phang,Aust. J. Chem. 32, 1149 (1979).
S. Phang,Aust. J. Chem. 30, 1605 (1977).
S. Phang,Malaysian J. Sci. 4(B), 81 (1976).
D. J. G. Ives and G. J. Janz,Reference Electrodes (Academic Press, New York, 1961).
H. Falkenhagen and E. L. Vernon,Philos. Mag. 14, 537 (1932).
D. E. Goldsack and R. C. Franchetto,Can. J. Chem. 55, 1062 (1977).
R. N. Goldberg and R. L. Nuttal,J. Phys. Chem. Ref. Data 7, 263 (1978).
L. A. Dunn,Trans. Faraday Soc. 62, 2348 (1966).
G. Perron, J. E. Desnoyers, and F. J. Millero,Can. J. Chem. 52, 3738 (1974).
F. J. Millero,Chem. Rev. 71, 147 (1971).
H. E. Wirth and F. K. Bangert,J. Phys. Chem. 76, 3488 (1972).
T. Shedlovsky and A. S. Brown,J. Am. Chem. Soc. 56, 1066 (1934).
J. M. Fortes, M. Mercier, and J. Molenat,J. Chem. Phys. 71, 164 (1974).
R. A. Robinson and R. H. Stokes,Electrolyte Solutions, 2nd edn. (Butterworths, London, 1965), p. 461.
R. A. Robinson and R. H. Stokes,Electrolyte Solutions, 2nd edn. (Butterworths, London, 1965), p. 463.
R. H. Stokes,Trans. Faraday Soc. 41, 642 (1945).
N. S. Gill and R. S. Nyholm,J. Chem. Soc., 3997 (1959).
W. Devine and B. M. Lowe,J. Chem. Soc. (A), 2113 (1971).
R. A. Robinson and R. H. Stokes,Electrolyte Solutions, 2nd edn. (Butterworths, London, 1965), pp. 457, 497.
T. L. Broadwater and D. F. Evans,J. Solution Chem. 3, 757 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phang, S., Stokes, R.H. Density, viscosity, conductance, and transference number of concentrated aqueous magnesium chloride at 25°C. J Solution Chem 9, 497–505 (1980). https://doi.org/10.1007/BF00647738
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00647738