Abstract
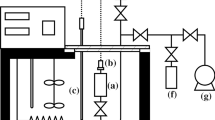
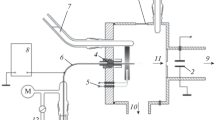
Heats of solution of nine electrolytes in 1,2-dichloroethane and of three electrolytes in 1,1-dichloroethane have been determined calorimetrically at various electrolyte concentrations and extrapolated to zero concentration to yield ΔH os values for these electrolytes. It is shown that values of ΔH ot for transfer from water to the dichloroethanes of 1∶1 electrolytes are often negative, so that these electrolytes can be more stable enthalpically in the less polar solvents. Combinations of the ΔH ot values with previously determined ΔG ot values yield values of ΔS ot for transfer of 1∶1 electrolytes from water to the dichloroethanes. These ΔS ot values are mostly very negative; they can be correlated very well by the method of Abraham, and in this way ΔS ot values for transfer of numerous other anions and cations have been predicted. The Ph4As+/Ph4B− convention yields single-ion entropies of transfer from water to the dichloroethanes in reasonable agreement with values calculated by the correspondence-plot method.
Similar content being viewed by others
References
C. M. Criss, R. P. Held, and E. Luksha,J. Phys. Chem. 72, 2970 (1968).
M. H. Abraham,J. Chem. Soc. Faraday Trans. 1,69, 1375 (1973).
B. G. Cox, G. R. Hedwig, A. J. Parker, and D. W. Watts,Aust. J. Chem. 27, 477 (1974).
G. R. Hedwig, D. A. Owensby, and A. J. Parker,J. Am. Chem. Soc. 97, 3888 (1975).
B. G. Cox,Chem. Soc. Annu. Rep. 70A, 249 (1973).
B. G. Cox and A. J. Parker,J. Am. Chem. Soc. 95, 6879 (1973).
C. M. Criss,J. Phys. Chem. 78, 1000 (1974).
B. Jakuszewski and S. Taniewska-Osińska,Soc. Sci. Lodz. Acta Chim. 4, 17 (1959);7, 31 (1961);8, 11 (1962).
M. H. Abraham,Chem. Commun., 888 (1972).
J. Strong and T. R. Tuttle, Jr.,J. Phys. Chem. 77, 533 (1973).
M. H. Abraham and A. F. Danil de Namor,J. Chem. Soc. Faraday Trans. 1 72, 955 (1976).
M. H. Abraham, R. J. Irving, and G. F. Johnston,J. Chem. Soc., 199 (1970).
Y.-C. Wu and H. L. Friedman,J. Phys. Chem. 70, 501 (1966).
C. de Visser and G. Somsen,Rec. Trav. Chim. 91, 942 (1972).
C. M. Criss and O. N. Bhatnagar,J. Phys. Chem. 73, 174 (1969).
T. S. Sarma, R. K. Mohanty, and J. C. Ahluwalia,Trans. Faraday Soc. 65, 2333 (1969).
E. M. Arnett, W. G. Bentrude, J. J. Burke, and P. McC. Duggleby,J. Am. Chem. Soc. 87, 1541 (1965).
L. L. Bright and J. R. Jezorek,J. Phys. Chem. 79, 800 (1975).
E. M. Arnett and D. R. McKelvey,J. Am. Chem. Soc. 88, 2598 (1966).
S. Villermaux and J. J. Delpuech,Bull. Soc. Chim. Fr., 2534 (1974).
O. Popovych,Anal. Chem. 46, 2009 (1974).
H. P. Bennetto, D. Feakins, and R. G. Bates, inHydrogen-Bonded Solvent Systems, A. K. Covington and T. Jones, eds. (Taylor and Francis, London, 1968), pp. 49 and 235.
C. M. Criss and M. Salomon, inPhysical Chemistry of Organic Solvent Systems, A. K. Covington and T. Dickenson, eds. (Plenum Press, New York, 1973), p. 253.
U. Mayer and V. Gutmann,Adv. Inorg. Chem. Radiochem. 17, 189 (1975).
I. A. Koppel and A. I. Paju,Org. React. (USSR) 11, 121 (1974).
R. M. Noyes,J. Am. Chem. Soc. 84, 513 (1962).
A. A. Maryott and E. R. Smith, Tables of Dielectric Constants of Pure Liquids, NBS Circular 514, 1951.
M. H. Abraham,Prog. Phys. Org. Chem. 11, 1 (1974).
B. B. Owen, R. C. Miller, C. E. Milner, and H. L. Cogan,J. Phys. Chem. 65, 2065 (1961).
D. J. Metz and A. Glines,J. Phys. Chem. 71, 1158 (1967); C. Carvajal, K. J. Tölle, J. Smid, and M. Szwarc,J. Am. Chem. Soc. 87, 5548 (1965).
V. Viti and P. Zampetti,Chem. Phys. 2, 233 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abraham, M.H., Danil de Namor, A.F. & Schulz, R.A. Heats of solution of 1:1 electrolytes in 1,2- and 1,1-dichloroethane and derived enthalpies and entropies of transfer of electrolytes from water to these solvents. J Solution Chem 5, 529–542 (1976). https://doi.org/10.1007/BF00647376
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00647376