Abstract
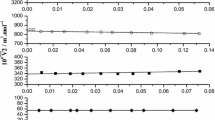
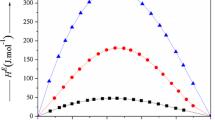
Calorimetric measurements have been made of the differential enthalpies of solution as a function of composition of both components in the binary systems tetraethyleneglycol octylether (C8E4)-water and polyethyleneglycol 400 (PEG)-water, as a function of composition, at three different temperatures. Heat capacity changes for dissolution were calculated from the temperature variation of the solution enthalpies. Excess enthalpies and excess heat capacities of mixing were calculated from the differential enthalpies of solution. All measurements on C8E4 were made above the critical micelle concentration (c.m.c.) so the results relate to C8E4 in aggregated form. The thermochemical properties of the C8E4 and PEG systems with water are similar. The differential solution enthalpy of the organic solute in pure water is fairly exothermic and then increases smoothly with increasing solute content. Likewise the solution enthalpy of water in pure C8E4 or PEG is fairly exothermic, but increases steadily to become zero at a water content corresponding to more than five water molecules per ethyleneoxide group. The measurements on the C8E4 system at 40°C were made close to the demixing temperature. The results are compared with previously reported results on the 2-butoxyethanol (BE)-water system.
Similar content being viewed by others
References
D. J. Mitchell, G. J. T. Tiddy, L. Waring, T. Bostock, and M. P. McDonald,J. Chem. Soc. Faraday Trans. I 79, 975 (1983).
J. C. Lang and R. D. Morgan,J. Chem. Phys. 73, 5849 (1980).
B. Andersson and G. Olofsson,Coll. Poly. Sci. 265, 318 (1987).
M. Corti, V. Degiorgio, and M. Zulauf,Phys. Rev. Lett. 48, 1617 (1982).
B. Andersson and G. Olofsson,J. Chem. Soc. Faraday Trans. I 84, 4087 (1988).
B. Andersson and G. Olofsson,J. Solution Chem. 17, 1169 (1988).
G. N. Malcolm and J. S. Rowlinson,Trans. Faraday Soc. 53, 921 (1957).
S. Sunner and I. Wadsö,Sci. Tools 13, 1 (1966).
I. Wadsö,Acta Chem. Scand. 22, 927 (1968).
A.-T. Chen and I. Wadsö,J. Biochem. Biophys. Methods 6, 307 (1982).
J. Suurkuusk and I. Wadsö,Chemica Scripta 20, 155 (1982).
M. Görman-Nordmark, J. Laynez, A. Schön, J. Suurkuusk, and I. Wadsö,J. Biochem. Biophys. Methods 10, 187 (1984).
D. Hallén, S.-O. Nilsson, W. Rothschild, and I. Wadsö,J. Chem. Thermodyn. 18, 429 (1986).
J. Suurkuusk and I. Wadsö,J. Chem. Thermodyn. 6, 667 (1974).
M. Zulauf and J. P. Rosenbusch,J. Phys. Chem. 87, 856 (1983).
J. Koller and E. Killmann,Makromol. Chem. 182, 3579 (1981).
H. Daoust and D. St-Cyr,Macromolecules 17, 596 (1984).
M. A. Stephens and W. S. Tamplin,J. Chem. Eng. News 24, 81 (1979).
R. Kjellander and E. Florin,J. Chem. Soc. Faraday Trans. I 77, 2053 (1981).
G. Karlström,J. Phys. Chem. 89, 4962 (1985).
R. Kjellander,J. Chem. Soc. Faraday Trans. II 78, 2025 (1982).
P.-G. Nilsson, H. Wennerström and B. Lindman,J. Phys. Chem. 87, 1377 (1983).
P.-G. Nilsson and B. Lindman,J. Phys. Chem. 87, 4756 (1983).
S.-O. Nilsson,J. Chem. Thermodyn. 18, 1115 (1986).
U. Onken,Z. Electrochem. 63, 321 (1959).
F. Elizalde, J. Gracia, and M. Costas,J. Phys. Chem. 92, 3565 (1988).
G. Olofsson, unpublished results.
N. Nichols, R. Sköld, C. Spink, J. Suurkuusk, and I. Wadsö,J. Chem. Thermodyn. 8, 1081 (1976).
S. Cabani, P. Gianni, V. Mollica, and L. Lepori,J. Solution Chem. 10, 563 (1981).
J. Biros, J. Pouchly, and A. Zivny,Makromol. Chem. 188, 379 (1987).
S. Saeki, N. Kuwahara, N. Nakata and M. Kaneko,Polymer 17, 685 (1976).
F. E. Bailey Jr. and R. W. Callard,J. Appl. Polym. Sci. 1, 56 (1959).
M. Herskowitz and M. Gottlieb,J. Chem. Eng. Data 30, 233 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andersson, B., Olofsson, G. Calorimetric study of binary systems of tetraethyleneglycol octylether and polyethyleneglycol with water. J Solution Chem 18, 1019–1035 (1989). https://doi.org/10.1007/BF00647261
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00647261