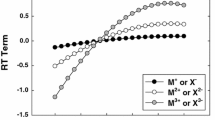
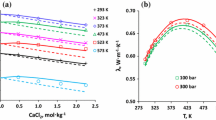
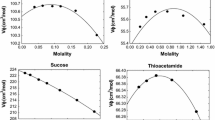
Abstract
Equations in the ion-interaction (Pitzer) system are derived for the volume change on mixing any combination of the sea salts NaCl, Na2SO4, MgSO4, MgCl2 at constant ionic strenth. For these mixings of different charge types, the equations include complex differences of pure electrolyte terms. Recently measured data for each of the pure electrolytes provide these pure electrolyte terms. Other recent measurements on the volume change on mixing are compared with values calculated from the equations. At 25°C there is no need to introduce the mixing terms based on differences in the interactions of ions of the same sign. At other temperatures, the agreement without the mixing terms is good, but significant improvement is obtained by inclusion of the binary mixing terms θCl,SO v4 and θ vNa,Mg . The equations and parameters can then predict the volumetric properties of any mixed solution of these salts over the range 0–100°C and to at least 3 mol-kg−1 ionic strength.
Similar content being viewed by others
References
K. S. Pitzer,J. Phys. Chem. 77, 268 (1973)
K. S. Pitzer,Theory: Ion Interaction Approach. In Activity Coefficients in Electrolyte Solutions (CRC Press, Boca Raton, FL, 1979), pp. 157.
P. S. Z. Rogers and K. S. Pitzer,J. Phys. Chem. Ref. Data 11, 15 (1982).
K. S. Pitzer,Reviews in Mineralogy 17, 97 (1987).
F. J. Millero, L. M. Connaughton, F. Vinokurova, and P. V. Chetirkin,J. Solution Chem. 14, 837 (1985).
L. M. Connaughton, M. S. Thesis, University of Miami, Florida (1985).
L. M. Connaughton, J. P. Hershey, and F. J. Millero,J. Solution Chem. 15, 989 (1986). The following corrections should be made in Table IV: for MgCl2, change the sign ofC 4 to + ande 2 to − for MgSO4,d 2=6.1103123E-1.
L. M. Connaughton and F. J. Millero,J. Solution Chem. 16, 491 (1987).
C. E. Harvie and J. H. Weare,Geochim. Cosmochim. Acta 44, 981 (1980).
C. E. Harvie, N. Møller, and J. H. Weare,Geochim. Cosmochim. Acta 48, 723 (1984).
B. S. Krumgalz and F. J. Millero,Marine Chem. 11, 209 (1982).
F. J. Millero and V. Thurmond,J. Solution Chem. 12, 401 (1983).
F. J. Millero,Geochim. Cosmochim. Acta 47, 2121 (1983).
F. J. Millero,Thalassia Jugoslavica 18, 253 (1982).
R. N. Roy, J. J. Gibbons, J. C. Peiper, and K. S. Pitzer,J. Phys. Chem. 87, 2365 (1983).
A. Kumar and G. Atkinson,J. Phys. Chem. 87, 5504 (1983).
A. Kumar,J. Chem. Eng. Data 31, 19 (1986).
A. Kumar,J. Chem. Eng. Data 32, 106 (1987).
A. Kumar,J. Chem. Eng. Data 33, 198 (1988).
R. C. Phutela and K. S. Pitzer,J. Chem. Eng. Data 31, 320 (1986).
P. P. S. Saluja, K. S. Pitzer, and R. C. Phutela,Can. J. Chem. 64, 1328 (1986).
R. C. Phutela, K. S. Pitzer, and P. P. S. Saluja,J. Chem. Eng. Data 32, 76 (1987).
D. J. Bradley and K. S. Pitzer,J. Phys. Chem. 83, 1599 (1979).
K. S. Pitzer,J. Solution Chem. 4, 249 (1975).
K. S. Pitzer,J. Phys. Chem. 87, 2360 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Connaughton, L.M., Millero, F.J. & Pitzer, K.S. Volume changes for mixing the major sea salts: Equations valid to ionic strength 3.0 and temperature 95°C. J Solution Chem 18, 1007–1017 (1989). https://doi.org/10.1007/BF00647260
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00647260