Abstract
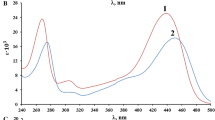
The visible absorption spectra of 4-(2-benzothiazolylazo)resorcinol (BTAR) and 4-(2-benzothiazolylazo)salicylic acid (BTAS) have been recorded in water-organic solvent mixtures in the pH range 0.5–12.0. The organic solvents used are methanol, ethanol,n-propanol, iso-propanol, acetone, dioxane and dimethyl formamide. The spectral changes have been explained in terms of shifts in equilibria among different molecular and ionic species of BTAR and BTAS existing in solution. The pK a values corresponding to the different ionization steps have been determined at 25°C and I=0.1M (KNO3) by graphic analysis of the absorbance-pH curves. The results obtained are discussed in terms of the molecular structure of the reagents and the nature of the organic co-solvent used.
Similar content being viewed by others
References
V. Chromy and L. Sommer,Talanta 14, 393, (1967).
L. Sommer, T. Sepel and V. M. Ivanov,Talanta 15, 949 (1968).
S. Shibata,Chelates in Analytical Chemistry, H. A. Flascka and A. J. Barnard, Jr. eds., Vol. 4. (Dekker, New York, 1972).
H. R. Hovind,Analyst 100, 769 (1975).
F. Garcia Montelongo, J. J. Arias, and F. Jimenez,Mikrochim. Acta 11, 349 (1983).
Ma. J. Sanchez, A. Francisco, F. Jimenez, and F. Garcia Montelongo,Talanta 36, 831 (1989).
C. P. Zhang, D. Y. Qi, and T. Z. Zhou,Talanta 29, 1119 (1982).
F. H. Pollard, G. Nicklers, T. J. Samuelson, and R. J. Anderson,J. Chromatogr. 16, 231 (1964).
A. I. Vogel,Practical Organic Chemistry 3rd edn, (Longmans, London, 1961).
A. I. Vogel,A Text Book of Quantitative Inorganic Analysis, (Longmans Green, London, 1975).
G. Douheret,Bull. Soc. Chim. Fr. 1412 (1967).
G. Douheret,Bull. Soc. Chim. Fr. 3122 (1968).
L. Sommer, V. Kuban and J. Havel,Folia Fac. Sci. Nat. Univ. Brno Chemia 7, 33 (1970).
F. J. C. Rossotti and H. Rossotti,The Determination of Stability Constants (McGraw-Hill, New York, 1961).
V. Kuban, L. Sommer, and J. Havel,Colln. Czech Chem. Comun. 40, 604 (1975).
L. G. Sillen and B. Warnqvist,Ark. Kemi 31, 377 (1969).
J. F. Coetzee and C. D. Richie,Solute-Solvent Interactions, (Marcel Dekker, New York, 1969).
A. Albert, R. Goldacre, and J. Philips,J. Chem. Soc. 2240 (1948).
L. Sommer and Y. M. Ivanov,Talanta 14, 171 (1967).
O. S. Hafez, Ph.D. Thesis, Alexandria University, Faculty of Science, 1990.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abu-Bakr, M.S., El-Shahawy, A.S. & Ahmed, S.M. Spectrophotometric study of acid-base equilibria of 4-(2-benzothiazolylazo)resorcinol and 4-(2-benzothiazolylazo)salicylic acid in water-organic solvent media. J Solution Chem 22, 663–675 (1993). https://doi.org/10.1007/BF00646785
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00646785