Abstract
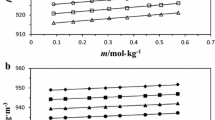
The enthalpies of solution of tetra-n-butylammonium bromide have been measured in mixtures of formamide (F) with water, N-methylformamide (NMF) with water, N,N-dimethylformamide (DMF) with water, F with NMF, DMF with F, and NMF with DMF at 25°C in the whole mole-fraction range. The enthalpies of solution vs composition profiles show a maximum value in the DMF-H2O and in the DMF-F systems. In the F-NMF and NMF-DMF mixturesn-Bu4NBr displays a nearly ideal behavior, whereas in the other solvent systems the excess enthalpy of solution deviates substantially from zero.
Similar content being viewed by others
References
G. Somsen, L. Weeda, and J. M. Los,J. Electroanal. Chem. Interfacial Electrochem. 29, 375 (1971).
G. Somsen and L. Weeda,J. Electroanal. Chem. Interfacial Electrochem. 31, 9 (1971).
C. de Visser and G. Somsen,Rec. Trav. Chim. 90, 1129 (1971).
C. de Visser and G. Somsen,J. Chem. Thermodyn. 4, 313 (1972).
C. de Visser and G. Somsen,Rec. Trav. Chim. 91, 942 (1972).
C. de Visser and G. Somsen,J. Chem. Thermodyn. 5, 147 (1973).
C. de Visser and G. Somsen,J. Chem. Soc. Faraday (I),69, 1440 (1973).
F. Franks and D. J. G. Ives,Quart. Rev. 20, 1 (1966).
E. M. Arnett and D. R. McKelvey,J. Am. Chem. Soc. 87, 1393 (1965).
E. M. Arnett, inPhysico-chemical Processes in Mixed Aqueous Solvents, F. Franks, ed. (Heineman, London, 1967) p. 105.
B. G. Cox,J. Chem. Soc. Perkin (II), 607 (1973).
R. K. Mohanty, T. S. Sarma, S. Subramanian, and J. C. Ahluwalia,Trans. Faraday Soc. 67, 305 (1971).
S. J. Bass, W. J. Nathan, R. M. Meighan, and R. H. Cole,J. Phys. Chem. 68, 509 (1964).
R. C. Paul, J. P. Singla, D. S. Gill, and S. P. Narula,Indian J. Chem. 9, 981 (1971).
M. Postel and J. Vedel,J. Electroanal. Chem. Interfacial Electrochem. 29, 69 (1971).
S. R. Gunn,J. Chem. Thermodyn. 2, 535 (1970).
R. B. Cassel and W. Y. Wen,J. Phys. Chem. 76, 1389 (1972).
C. E. Vanderzee and D. L. King,J. Chem. Thermodyn. 4, 675 (1972).
T. S. Sarma and J. C. Ahluwalia,Trans. Faraday Soc. 67, 2528 (1971).
M. J. Mastroianni, M. J. Pikal, and S. Lindenbaum,J. Phys. Chem. 76, 3050 (1972).
R. H. Wood, private communication.
P. Rohdewald and M. Möldner,J. Phys. Chem. 77, 373 (1973).
J. F. Hinton and K. H. Ladner,J. Magn. Res. 6, 586 (1972).
J. F. Hinton and K. H. Ladner,Spectrochim. Acta 28A, 1731 (1972).
P. Assarsson and F. R. Eirich,J. Phys. Chem. 72, 2710 (1968).
T. Oncescu and E. Jurconi,Rev. Roumaine Chim. 16, 1033 (1971).
S. Saphon and H. J. Bittrich,Z. Physik. Chemie (Leipzig) 252, 113 (1973).
Z. Kozlowski,Soc. Sci. Lodz. Acta Chim. 16, 17 (1971).
R. C. Peterson,J. Phys. Chem. 64, 184 (1960).
E. P. Egan and B. B. Luff,J. Chem. Eng. Data 11, 194 (1966).
H. Peters and E. Tappe,Monatsber. Deut. Akad. Wiss. Berlin9, 692 (1967).
S. Taniewska-Osinska, Z. Kozlowski, and M. Woldan,Soc. Sci. Lodz. Acta Chim. 16, 25 (1971).
E. N. Vasenko and S. M. Dubrovsky,Zh. Fiz. Khim. 27, 281, 1387 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Visser, C., Somsen, G. Enthalpies of solution of tetra-n-butylammonium bromide in binary mixtures of water, formamide, N-methylformamide, and N,N-dimethylformamide. J Solution Chem 3, 847–855 (1974). https://doi.org/10.1007/BF00645690
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00645690