Summary
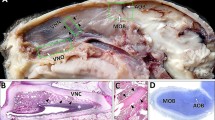
Components of the γ-glutamyl cycle, including thiols, glutathione (GSH) and γ-glutamyl transpeptidase (γ-GT), were localized in the nasal mucosae of rats using histochemical and immunohistochemical methods. In olfactory mucosa, thiols were widely distributed, with intense staining in the mucociliary complex (MC), basal cells, acinar cells of Bowman's glands (BG), and olfactory nerve bundles, and with moderate staining in olfactory receptor neurons (ORNs). GSH was localized in MC, BG acinar cells, nerve bundles and, to a lesser extent, in ORNs. γ-GT immunoreactivity was restricted to the MC and to basolateral and apical membranes of BG acinar and duct cells. The basolateral membrane of BG acinar cells, located in close association with blood vessels and connective tissue, showed granule-like immunoreactivity. Inrespiratory mucosa, all three compounds were localized in the MC and acinar cells of respiratory glands (RG). In the MC, γ-GT immunoreactivity was associated primarily with brush borders of ciliated cells. Granular immunoreactivity was also apparent in the supranuclear region of RG acinar cells. These results demonstrate that components of the γ-glutamyl cycle are localized in olfactory and respiratory glands, and that they are secreted into the mucus, where they may mediate perireceptor events such as detoxification and/or solubilization of air-borne xenobiotics, toxicants and odorants.
Similar content being viewed by others
References
Alpers DH (1987) Digestion and absorption of carbohydrates and proteins. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 2nd edn. Raven Press, New York, pp 1469–1486
Asghar K, Reddy BG, Krishna H (1975) Histochemical localization of glutathione in tissues. J Histochem Cytochem 23:774–779
Baker H, Spencer RF (1986) Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res 63:461–473
Buchheit K, Walters E, Maruniak J (1991) Unilateral naris closure alters cytochrome P-450 expression in adult mouse olfactory mucosa (abstract). Chem Senses 16:506
Chieco P, Boor PJ (1983) Use of low temperatures for glutathione histochemical stain. J Histochem Cytochem 31:975–976
Cohen G (1983) The pathology of Parkinson's disease: biochemical aspects of dopamine neuron senescence. J Neural Transm [Suppl] 19:213–217
Cornell JS, Meister A (1976) Glutathione and γ-glutamyl cycle enzymes in crypt and villus tip cells of rat jejunal mucosa. Proc Natl Acad Sci USA 73:420–422
Dahl AR (1988) The effect of cytochrome P-450-dependent metabolism and other enzyme activities on olfaction. In: Margolis FL, Getchell TV (eds) Molecular neurobiology of the olfactory system. Plenum Press, New York, pp 51–70
Dear TN, Campbell K, Rabbitts TH (1991) Molecular cloning of putative odorant-binding and odorant-metabolizing proteins. Biochemistry 30:10376–10382
Delaleu JC, Holley A (1980) Modification of transduction mechanisms in the frog's olfactory mucosa using a thiol reagent as olfactory stimulus. Chem Senses Flav 5:205–218
Deleve LD, Kaplowitz N (1990) Importance and regulation of hepatic glutathione. Semin Liver Dis 10:251–266
Deml E, Oesterle D (1980) Histochemical demonstration of enhanced glutathione content in enzyme-altered islands induced by carcinogens in rat liver. Cancer Res 40:490–491
Ding X, Coon MJ (1990) Immunochemical characterization of multiple forms of cytochrome P-450 in rabbit nasal microsomes and evidence for tissue-specific expression of P-450s NMa and NMb. Mol Pharmacol 37:489–496
Ding X, Porter TD, Peng HM, Coon MJ (1991) cDNA and derived amino acid sequence of rabbit nasal cytochrome P-450NMb (P450IIG1), a unique isozyme possibly involved in olfaction. Arch Biochem Biophys 285:120–125
Foster JD, Getchell ML, Getchell TV (1992) Ultrastructural localization of sialylated glycoconjugates in cells of the salamander olfactory mucosa using lectin cytochemistry. Cell Tissue Res 267:113–124
Gainer H, Kosower NS (1980) Histochemical demonstration of thiols and disulfides by the fluorescent labeling agent, monobromobimane: an application to the hypothalamo-neurohypophysial system. Histochemistry 68:309–315
Garvey TQ III, Hyman PE, Isselbacher KJ (1976) γ-Glutamyl transpeptidase of rat intestine: Localization and possible role in amino acid transport. Gastroenterology 71:778–785
Getchell ML, Gesteland RC (1972) The chemistry of olfactory reception: Stimulus-specific protection from sulfhydryl reagent inhibition. Proc Natl Acad Sci USA 69:1494–1498
Getchell TV, Margolis FL, Getchell ML (1984) Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol 23:317–345
Harisch G, Meyer W (1985) Studies on tissue distribution of glutathione and on activities of glutathione-related enzymes after carbon tetrachloride-induced liver injury Res Comm Chem Pathol Pharmacol 47:399–414
Keller A, Margolis FL (1975) Immunological studies of the rat olfactory marker protein. J Neurochem 31:1101–1106
Kirstein CL, Coopersmith R, Bridges RJ, Leon M (1991) Glutathione levels in olfactory and non-olfactory neural structures of rats. Brain Res 543:341–346
Koob M, Dekant W (1991) Bioactivation of xenobiotics by formation of toxic glutathione conjugation. Chem Biol Interact 77:107–136
Lazard D, Tal N, Rubinstein M, Khen M, Lancet D, Zupko K (1990) Identification and biochemical analysis of novel olfactory-specific cytochrome P-450IIA and UDP-glucuronosyl transferase. Biochemistry 29:7433–7440
Lazard D, Zupko K, Poria Y, Nef P, Lazarovits J, Horn S, Khen M, Lancet D (1991) Odorant signal termination by olfactory UDP glucuronosyl transferase. Nature 349:790–793
Marathe GV, Nash B, Haschenmeyer, Tate SS (1979) Ultrastructural localization of γ-glutamyl transpeptidase in rat kidney and jejunum. FEBS Lett 107:436–440
Margolis FL, Grillo M (1977) Axoplasmic transport of carnosine (beta-alanyl-L-histidine) in the mouse olfactory pathway. Neurochem Res 2:507–519
Meister A (1973) On the enzymology of amino acid transport. Science 80:33–39
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760
Meister A, Tate SS (1976) Glutathione and related γ-glutamyl compounds: biosynthesis and utilization. Ann Rev Biochem 45:559–604
Meister A, Griffith OW, Novogrodsky A, Tate SS (1980) New aspects of glutathione metabolism and translocation in mammals. In: Sulfur in biology, Ciba Foundation symposium 72 (new series), Exerpta Medica. Elsevier, North Holland, Amsterdam, pp 135–161
Menevse A, Dodd GH, Poynder TM, Squirrel D (1977) A chemical-modification approach to the olfactory code. Biochem Soc Trans 5:191–194
Menevse A, Dodd G, Poynder TM (1978) A chemical-modification approach to the olfactory code. Studies with a thiol-specific reagent. Biochem J 176:845–854
Nash B, Tate SS (1982) Biosynthesis of rat renal γ-glutamyl transpeptidase. Evidence for a common precursor of the two subunits. J Biol Chem 257:585–588
Nash B, Tate SS (1984) In vitro translation and processing of rat kidney γ-glutamyl transpeptidase. J Biol Chem 259:678–685
Pace U, Lancet D (1987) Molecular mechanisms of vertebrate olfaction: Implications for pheromone biochemistry. In: Prestwich GD, Blomquist GJ (eds) Pheromone biochemistry. Academic Press, New York, pp 529–546
Pfaller W, Gstraunthaler G, Kotanko P, Wolf H, Curthoys NP (1984) Immunocytochemical localization of γ-glutamyl-transferase on isolated renal cortical tubular fragments. Histochemistry 80:289–293
Rama Krishna NS, Getchell ML, Getchell TV (1992) Differential distribution of γ-glutamyl cycle molecules in the vomeronasal organ of rats. Neuro Report 3:551–554
Randall HW, Bogdanffy MS, Morgan KT (1987) Enzyme histochemistry of the rat nasal mucosa embedded in cold glycol methacrylate. Am J Anat 179:10–17
Ravindranath V, Shivakumar BR, Anandatheerthavarada HK (1989) Low glutathione levels in brain regions of aged rats. Neurosci Lett 101:187–190
Saneto RP, Awasthi YC, Srivastava SK (1982) Glutathione S-transferase of the bovine retina. Biochem J 205:213–217
Shienvold FL, Alejandro R, Latif Z, Mintz DH (1986) Human pancreatic cell surface antigens with homologous expression in liver, sweat glands, and other exocrine tissue. Pancreas 1:385–392
Shiozawa M, Yamashita S, Aiso S, Yasuda K (1989) A monoclonal antibody against human kidney gamma-glutamyl transpeptidase: preparation, immunochemical and immunohistochemical characterization. J Histochem Cytochem 37:1053–1061
Shirley S, Polak EH, Dodd G (1983) Chemical modification studies on rat olfactory mucosa using a thiol-specific reagent and enzymatic iodination. Eur J Biochem 132:485–494
Slivka A, Mytilineou C, Cohen G (1987) Histochemical evaluation of glutathione in brain. Brain Res 409:275–284
Spater HW, Poruchynsky MS, Quintana N, Inoue M, Novikoff AB (1982) Immunohistochemical localization of γ-glutamyl-transferase in rat kidney with protein A-horseradish peroxidase. Proc Natl Acad Sci USA 79:3547–3550
Starcevic S, Muruganandam A, Mutus B, Zielinski B (1991) The use of monobromobimane to study glutathione in the rainbow trout olfactory organ (abstract). Chem Senses 16:586
Stefanini M, De Martino C, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173–174
Suzuki Y, Takeda M (1991) Basal cells in the mouse olfactory epithelium after axotomy: immunohistochemical and electronmicroscopic studies. Cell Tissue Res 266:239–245
Takahashi H (1984) Scanning electron microscopy of the rat exocrine pancreas. Arch Histol Jpn 47:387–404
Tate SS (1980) Enzymes of mercapturic acid formation. In: Jakoby WB (ed) Enzymatic basis of detoxication, vol II. Academic Press, New York, pp 95–120
Tate SS, Meister A (1976) Subunit structure and isozymic forms of γ-glutamyl transpeptidase. Proc Natl Acad Sci USA 73:2599–2603
Tate SS, Meister A (1981) γ-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem 39:357–368
Viña J, Viña JR (1983) Role of γ-glutamyltranspeptidase in the regulation of aminoacid uptake by mammary gland of lactating rat. In: Larsson A, Orrenius S, Holmgren A, Mannervik B (eds) Functions of glutathione biochemical physiological, toxicological and clinical aspects. Raven Press, New York, pp 23–30
Viña J, Purtes IR, Estrela JM, Viña JR, Galbis JL (1981) Involvement of γ-glutamyltransferase in amino-acid uptake by the lactating mammary gland of the rat. Biochem J 194:99–102
Yamamoto M (1976) An electron microscopic study of the olfactory mucosa in the bat and rabbit. Arch Histol Jap 38:359–412
Yamashita K, Hitoi A, Matsuda Y, Tsuji A, Katunuma N, Kobata A (1983) Structural studies of the carbohydrate moieties of rat kidney γ-glutamyltranspeptidase. J Biol Chem 258:1098–1107
Yasuda K, Yamashita S, Aiso S, Shiozawa M, Komatsu T (1986) Immunohistochemical study of γ-glutamyl transpeptidase with monoclonal antibodies. I. Preparation and characteristics of monoclonal antibodies to γ-glutamyl transpeptidase. Acta Histochem Cytochem 19:589–594
Yasuda K, Yamashita S, Shiozawa M (1989) An experimental study on the application of microwave fixation to immunohistochemical studies. Acta Histochem Cytochem 22:91–98
Yasuda K, Shiozawa M, Aiso S, Taniguchi S, Yamashita S (1990a) Distribution of gamma-glutamyl transpeptidase in human pancreas: immunohistochemical study with a monoclonal antibody. J Histochem Cytochem 38:339–350
Yasuda K, Shiozawa M, Yamashita S, Aiso S (1990b) Localization of γ-glutamyl transpeptidase in human sweat glands: An immunohistochemical study using a monoclonal antibody. J Histochem Cytochem 38:1433–1444
Zupko K, Poria Y, Lancet D (1991) Immunolocalization of cytochromesP-450olf1 andP-450olf2 in rat olfactory mucosa. Eur J Biochem 196:51–58
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rama Krishna, N.S., Getchell, M.L., Tate, S.S. et al. Glutathione and γ-glutamyl transpeptidase are differentially distributed in the olfactory mucosa of rats. Cell Tissue Res. 270, 475–484 (1992). https://doi.org/10.1007/BF00645049
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00645049