Abstract
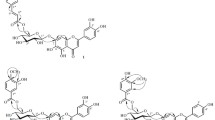
From the epigeal part ofHaplophyllum perforatum we have isolated the coumarins scopoletin (I), scopoletin 7-0-β-D-glucopyranoside (II) and the new coumarin glycoside haploperoside A (III), mp 212–213°C, [α] 22D −37° (c 0.24, CH3OH). The acid hydrolysis of (III) formed (I) and the monosaccharides D-glucose and L-rhamnose. Partial hydrolysis of (III) with 10% acetic acid led to (II) and L-rhamnose. On the basis of the results of a study of UV, IR, and PMR spectra, and also periodate oxidation and polarimetric analysis the structure of 6-methoxy-7-[0-α-L-rhamnopyranosyl-(2→1)-β-D-glucopyranosyloxy] coumarin has been established for (III). Details of the IR, UV, PMR, and mass spectra are given.
Similar content being viewed by others
Literature cited
M. G. Pimenov, List of Plants that are Sources of Coumarin Compounds [in Russian], Leningrad (1971), p. 43.
S. Yu. Yunusov, Alkaloids [in Russian], Tashkent (1974), p. 181.
G. A. Kuznetsova, Natural Coumarins and Furocoumarins [in Russian], Leningrad (1967), p. 74.
L. I. Kosheleva and G. K. Nikonov, Farmatsiya, No. 4, 78 (1969).
M. E. Perel'son, Yu. N. Sheinker, and A. A. Savina, The Spectra and Structures of Coumarins, Chromones, and Xanthones [in Russian], Moscow (1975), p. 9.
T. A. Sergienko, L. S. Kazarnovskii, and V. I. Litvinenko, Khim. Prir. Soedin., 166 (1966).
B. N. Stepanenko, The Chemistry and Biochemistry of Carbohydrates (Polysaccharides) [in Russian], Moscow (1978), p. 17.
V. I. Litvinenko and V. A. Makarov, Khim. Prir. Soedin., 366 (1969).
T. A. Sergienko, L. S. Kazarnovskii, and V. I. Litvinenko, Farmatsiya, 34 (1967).
B. N. Stepanenko, The Chemistry and Biochemistry of Carbohydrates (Monosaccharides) [in Russian], Moscow (1977), p. 90.
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer, New York (1970), p. 268.
V. N. Spiridonov, I. P. Kovalev, and A. P. Prokopenko, Khim. Prir. Soedin., 5 (1969).
R. M. Horowitz and B. Gentili, Tetrahedron,19, 773 (1963).
J. B. Harborne and T. J. Mabry, The Flavonoids, Chapman and Hall, London (1975), p. 398.
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenni, No. 2, pp. 168–172, March–April, 1980.
Rights and permissions
About this article
Cite this article
Yuldashev, M.P., Batirov, E.K. & Malikov, V.M. Coumarin glycosides ofHaplophyllum perforatum . Chem Nat Compd 16, 125–128 (1980). https://doi.org/10.1007/BF00638768
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00638768