Summary
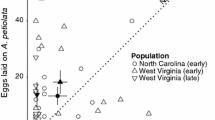
The eastern tiger swallowtail butterfly,Papilio glaucus, is the most polyphagous of all Papilionidae species. While diverse larval detoxication abilities are known for bothPapilio glaucus and the closely relatedP. canadensis, the factors affecting oviposition preferences in adult females are unknown. These congeneric species were studied to determine the extent of oviposition mistakes on toxic plants. We were also interested in comparing the geographic patterns of variation in oviposition preferences and the genetic basis of these differences. We conducted oviposition three-choice studies with the Canadian tiger swallowtail butterfly,Papilio canadensis, and the Eastern tiger swallowtail,Papilio glaucus, giving them the choice of (1) tuliptree,Liriodendron tulipifera, which is toxic to virtually all populations (P. canadensis) north of the Great Lakes Region of North America, (2) quaking aspen,Populus tremuloides, which is toxic to essentially all populations (P. glaucus) south of the Great Lakes, and (3) black cherry,Prunus serotina, which is an excellent foodplant for all members of thePapilio glaucus group, but which does not occur at latitudinal extremes of North America (in Alaska and most of Canada or the southern half of Florida). Handpaired interspecific hybrids were tested under the same experimental design to evaluate the possibility of sexlinked oviposition behavior. There was considerable variability in the choice of plants by individual butterflies, but a general trend suggesting that the females of each species had a lower preference for the plant toxic to their larvae. More than 6000 oviposition bouts were counted from 37 differentp. canadensis and 54p. glaucus females along a latitudinal transect of approximately 5000 km from Alaska south through the Great Lakes hybrid zone region to southern Florida. While not exceptionally high anywhere, the preference for aspen (Salicaceae) declined precipitously in central Michigan (45° N latitude) and remained very low (5–12%) in all locations southward to Florida, whereas we observed a reciprocal trend in preference for tuliptree (Magnoliaceae) which was greatest in Florida (87% of all eggs) and steadily declined northward across the Great Lakes region. Cherry was selected in these 3-choice tests at a relatively consistent and low frequency at all latitudes. Fixed allele differences in sex-linked (LDH and PGD) and autosomal (HK) electromorphs are known forP. glaucus andP. canadensis. Our electrophoretic data suggest that the preference of an individual female for aspen is not simply a characteristic of the northern species (P. canadensis) but can occur inP. glaucus females. The reciprocal situation is also evident in Northern Michigan and Wisconsin females (scored electrophoretically and morphologically asP. canadensis) which sometimes exhibit a clear preference for the toxic tuliptree. In fact, Alaskan populations ofP. canadensis chose tuliptree for about 52% of their eggs, even though none of their offspring has ever survived on this plant species in laboratory studies. We conclude that even with distinctive latitudinal trends, a considerable amount of local variation in relative oviposition preference exists among individuals of these polyphagous species. BothP. glaucus andP. canadensis will lay eggs on toxic plants. It appears that factors selecting against oviposition on toxic tuliptrees have been minimal (relative to other factors) in Alaska and somewhat stronger in the Great Lakes hybrid zone. It is in this zone of contact with tuliptree where selection against theP. canadensis populations ovipositing on tuliptree may be strong due to high larval mortality when such natural “mistakes” are made. We do not know whether behavioral preference changes evolutionarily preceded or followed the development of specific physiological detoxication abilities for tuliptree or quaking aspen. However, for bothP. canadensis andP. glaucus the occurrence of “oviposition mistakes” on toxic plants by adults extends geographically well beyond the larval detoxication abilities of their offspring. Hybrid female offspring of pairings with Michigancanadensis females andglaucus males show distinct preferences for tuliptree, suggesting that oviposition may be controlled by a factor (or factors) on the sex chromosome. Unfortunately we were unable to obtain reciprocal hybrids to evaluate the possibility of sex-linked aspen preference.
Similar content being viewed by others
References
Atsatt PR (1981) Lycaenid butterflies and ants: selection for enemy-free space. Am Nat 118:638–654
Berenbaum M (1981) An oviposition ‘mistake’ byPapilio glaucus Papilionidae. J Lepid Soc 35:75
Bossart JL, Scriber JM (1992) Genetic variation in oviposition preference in the tiger swallowtail butterfly: interspecific, interpopulation and interindividual comparisons. In: Scriber JM, Tsubaki Y, Lederhouse RC (eds) The swallowtail butterflies: their ecology and evolutionary biology. Cornell University Press, NY (in press)
Brower LP (1958) Larval foodplant specificity in butterflies of thePapilio glaucus group. Lepidoptera News 12:103–114
Butlin R (1990) Swallowtail performance. Nature 344:716
Chew FS (1977) Coevolution of pierid butterflies and their cruciferous foodplants. II. The distribution of eggs on potential foodplants. Evolution 31:568–579
Courtney SP (1981) Coevolution of pierid butterflies and their cruciferous foodplants. III.Anthocharis cardamines (L.) survival, development and oviposition on different host plants. Oecologia 51:91–96
Courtney SP, Courtney S (1982) The edge-effect in butterfly oviposition: causality inAnthocharis cardamines. Ecol Entomol 7:131–137
Courtney SP, Kibota TT (1990) Mother doesn't know best: selection of hosts by ovipositing insects. In: Bernays EA (ed) Insect-Plant Interactions. Vol 2:161–188, CRC Press
Courtney SP, Chen GK, Gardner A (1989) A general model for individual host selection. Oikos 55:55–65
Curtis JT (1959) The vegetation of Wisconsin. University of Wisconsin Press, Madison, Wisconsin, USA
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71:124–137
Dethier VG (1954) Evolution of feeding preferences in phytophagous insects. Evolution 8:33–54
Dowell RV, Scriber JM, Lederhouse RC (1990) Survival ofPapilio rutulus (Lepidoptera: Papilionidae) larvae on 38 potential hostplants. Pan Pacific Entomologist 66:140–146
Feeny PP (1990) Chemical constraints on the evolution of swallowtail butterflies. In: Price PW, Lewinsohn TM, Benson WW, Fernandes GW (eds) Herbivory: Tropical and temperate perspectives. John Wiley, NY, pp 315–340
Feeny P, Rosenberry L, Carter M (1983) Chemical aspects of oviposition behavior in butterflies. In: Ahmad S (ed) Herbivorous insects; Host-seeking behavior and mechanisms. Academic Press, NY, pp 27–76
Feeny P, Stadler E, Ahman I, Carter M (1989) Effects of plant oder on oviposition by the black swallowtail butterfly,Papilio polyxenes (Lepidoptera: Papilionidae). J Insect Behav 2:803–827
Fitt GP (1986) The influence of a shortage of hosts on the specificity of oviposition behavior in species ofDacus (Diptera: Tephrididae). Physiol Entomol 11:133–143
Futuyma DJ (1987) The role of behavior in host-associated divergence in herbivorous insects. In: Huettel M (ed) Evolutionary genetics of invertebrate behavior. Plenum, NY, pp 295–302
Futuyma DJ (1989) Macroevolutionary consequences of speciation: inferences from phytophagous insects. In: Otte D, Endler JA (eds) Speciation and its consequences. Sinauer, Sunderland, MA, pp 557–578
Futuyma DJ, Peterson S (1985) Genetic variation in the use of resources by insects. Annu Rev Entomol 30:217–238
Futuyma DJ, Cort RP, Noordwijky IV (1984) Adaptation to host plants in the fall cankerwormAlsophila pometaria and its bearing on the evolution of host affiliation in phytophagous insects. Am Nat 123:257–296
Gould F (1988) Genetics of pairwise and multispecies plant-herbivore coevolution. In: Spencer KC (ed) Chemically mediated coevolution. Academic Press, NY, pp 13–15
Grossmuller DW, Lederhouse RC (1985) Oviposition site selection: An aid to rapid growth and development in the tiger swallowtail,Papilio glaucus. Oecologia 66:68–73
Grossmuller DW, Lederhouse RC (1987) The role of nectar source distribution in habitat use and oviposition by the tiger swallowtail butterfly. J Lepid Soc 41:159–165
Hagen RH, Scriber JM (1989) Sex-linked diapause, color, and allozyme loci inPapilio glaucus: linkage, analysis and significance in a hybrid zone. Heredity 80:179–185
Hagen RH, Scriber JM (1991) Systematics of thePapilio glaucus andP. troilus species groups (Lepidoptera: Papilionidae): inferences from allozymes. Ann Entomol Soc Am (in press)
Hagen RH, Scriber JM (1992) Sex chromosomes and speciation in thePapilio glaucus group. In: Scriber JM, Tsubaki T, Lederhouse RC (eds) The swallowtail butterflies: their ecology and evolutionary biology. Cornell University Press, NY. Chapter 23 (in press)
Hagen RH, Lederhouse RC, Bossart J, Scriber JM (1991)Papilio canadensis is a species. J Lepid Soc (in press)
Harris H, Hopkinson DA (1978) Handbook of enzyme electrophoresis in human genetics. American Elsevier, New York
Jaenike J (1988) Effects of early adult experience on host selection in insects: some experimental and theoretical results. J Insect Behav 1:3–15
Jaenike J (1989) Genetic population structure ofDrosophila tripunctata: patterns of variation and covariation of traits affecting resource use. Evolution 43:1467–1482
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Janzen DJ (1985) A hostplant is more than its chemistry. Ill Nat Hist Surv Bull 33:141–174
Lindroth RL, Scriber JM, Hsia MTS (1988) Chemical ecology of the tiger swallowtail: Mediation of host use by phenolic glycosides. Ecology 69:814–822
Luebke H, Scriber JM, Yandell BS (1988) Use of multivariate discriminant analysis of male wing morphometrics to delineate a hybrid zone forPapilio glaucus glaucus andP. g. canadensis. Am Midl Nat 119:366–379
Miller JS (1987) Host-plant relationships in the Papilionidae (Lepidoptera): parallel cladogenesis or colonization? Cladistics 3:105–120
Miller J, Strickler K (1984) Insect/plant interactions — finding and assessing host plants. In: Bell W, Carde R (eds) Chemical ecology of insects. Chapman and Hill, London, pp 127–157
Ng D (1988) A novel level of interactions in plant-insect systems. Nature 334:611–612
Nishida R, Fukami H (1989) Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly,Atrophaneura alcinous. J Chem Ecol 15:2565
Ohsugi T, Nishida R, Fukami H (1985) Oviposition stimulant ofPapilio xuthus, a citrus-feeding swallowtail butterfly. Agric Biol Chem 49:1897–1900
Papaj DR (1986) Interpopulation differences in host preference and the evolution of learning in the butterfly,Battus philenor. Evolution 40:518–530
Papaj DR, Rausher MD (1987) Components of conspecific host discrimination behavior in the butterfly,Battus philenor. Ecology 68:245–253
Pilson D, Rausher MD (1988) Clutch size adjustment by a swallowtail butterfly. Nature 333:361–363
Price PW, Westoby M, Rice B, Atsatt PR, Fritz RS, Thompson JN, Mobley K (1986) Parasite mediation in ecological interactions. Annu Rev Ecol Systematics 17:487–505
Rausher MD (1980) Host abundance, juvenile survival, and oviposition preference inBattus philenor. Evolution 34:342–355
Rausher MD (1983) Alteration of oviposition behavior byBattus philenor butterflies in response to variation in host-plant density. Ecology 64:1028–1034
Rausher MD, Papaj DR (1983) Host plant selection byBattus philenor butterflies: Evidence for individual differences in foraging behavior. Anim Behav 31:341–347
Richardson BJ, Baverstock PR, Adams M (1986) Allozyme electrophoresis. Academic Press, Orlando, Florida
Robinson R (1971) Lepidoptera genetics. Pergamon Press, Oxford
Rockey SJ, Hainze JH, Scriber JM (1987) Evidence of sex-linked diapause response inPapilio glaucus subspecies and their hybrids. Physiol Ecol 12:181–184
Schneider JC, Roush RT (1986) Genetic differences in oviposition preferences between 2 populations ofHeliothis virescens. In: Huettel MD (ed) Evolutionary genetics of invertebrate behavior. Plenum NY, pp 163–171
Scriber JM (1982) Foodplants and speciation in thePapilio glaucus group. In: Visser JH, Minks AK (eds) Proc 5th International Symp on Insect Plant Relationships, PUDOC, Wageningen, Netherlands, pp 307–314
Scriber JM (1984) Larval foodplant utilization by the World Papilionidae (Lepidoptera): Latitudinal gradients reappraised. Tokurana (Acta Rhopalocerologica) 2:1–50
Scriber JM (1986a) Allelochemicals and alimentary ecology: Heterosis in a hybrid zone? In: Brattsten L, Ahmed S (eds) Molecular mechanisms in insect plant associations. Plenum Press, NY, pp 43–71
Scriber JM (1986b) Origins of the regional feeding abilities in the tiger swallowtail butterfly: Ecological monophagy and thePapilio glaucus australis subspecies in Florida. Oecologia 71:94–103
Scriber JM (1988) Tale of the tiger: Beringial biogeography, bionomial classification, and breakfast choices in thePapilio glaucus complex of butterflies. In: Spencer KC (ed) Chemical mediation of coevolution. Academic Press, NY, pp 240–301
Scriber JM, Ayres MP (1990) New foodplant and oviposition records for the tiger swallowtail butterfly,Papilio glaucus canadensis in Alaska. Great Lakes Entomol 23:145–147
Scriber JM, Feeny PP (1979) Growth of herbivorous caterpillars in relation to feeding specialization and to growth form of their food plants. Ecology 60:829–850
Scriber JM, Lederhouse RC (1991) Thermal units as a resource dictating geographic patterns of feeding specialization of insect herbivores. In: Hunter MD, Ohguishi T, Price PW (eds) Effect of Resource Distribution on Animal/Plant Interactions. Academic Press (in press)
Scriber JM, Lintereur GL, Evans MH (1982) Foodplant suitabilities and a new oviposition record forPapilio glaucus canadensis (Lepidoptera: Papilionidae) in northern Wisconsin and Michigan. Great Lakes Entomol 15:39–46
Scriber JM, Lindroth RL, Nitao J (1989) Differential toxicity of a phenolic glycoside from quaking aspen leaves toPapilio glaucus subspecies, their hybrids, and backcrosses. Oecologia 81:186–191
Scriber JM, Lederhouse RC, Hagen RH (1991) Foodplants and evolution withinPapilio glaucus andPapilio troilus species groups (Lepidoptera: Papilionidae). In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Wiley NY, pp 341–373
Singer MC (1982) Quantification of host preference by manipulation of oviposition behavior in the butterflyEuphydryas editha. Oecologia 52:224–229
Singer MC (1983) Determinants of multiple host use by a phytophagous insect population. Evolution 37:389–403
Singer MC (1986) The definition and measurement of oviposition preference in plant-feeding insects. In: Miller J, Miller TA (eds) Insect-plant relations. Springer Verlag, NY, pp 65–94
Singer MC, Ng D, Thomas CD (1988) Heritability of oviposition preference and its relationship to offspring performance within a single insect population. Evolution 42:977–985
Singer MC, Thomas CD, Billington HL, Parmesan C (1989) Variation among conspecific insect populations in the mechanistic basis of diet breadth. Anim Behav 37:751–759
Snedecor GW, Cochran WG (1967) Statistical methods. Iowa State Univ. Press, Ames, IA
Stanton ML (1984) Short term learning and the searching accuracy of egg-laying butterflies. Anim Behav 32:33–40
Stanton ML, Cook RE (1983) Sources of intraspecific variation in host plant seeking behavior ofColias butterflies. Oecologia 60:365–370
Straatman R (1962) Notes on certain Lepidoptera ovipositing on plants which are toxic to their larvae. J Lepid Soc 16:99–103
Tabashnik BE (1983) Host range evolution: the shift from native legume hosts to alfalfa by the butterfly,Colias philodice eriphyle. Evolution 37:150–162
Thomas CD, Singer MC (1987) Variation in host preference affects movement patterns within a butterfly population. Ecology 68:1262–1267
Thompson JN (1988a) Evolutionary genetics of oviposition preference in swallowtail butterflies. Evolution 42:1223–1234
Thompson JN (1988b) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89
Thompson JN, Wehling W, Podolsky R (1990) Evolutionary genetics of host use in swallowtail butterflies. Nature 344:148–150
Tong ML, Shapiro AM (1989) Genetic differentiation among California populations of the Anise swallowtail butterfly,Papilio zelicaon Lucas. J Lepid Soc 43:217–228
Via S (1986) Genetic covariance between oviposition preference and larval performance in an insect herbivore. Evolution 39:505–523
Waldvogel M, Gould F (1990) Variation in oviposition preference ofHeliothis virescens in relation to macroevolutionary patterns of Heliothine host range. Evolution 44:1326–1337
Watanabe M (1982) Leaf structure ofZanthoxylum ailanthoides Sieb & Zucc. (Rutales: Rutaceae) affecting the mortality of a swallowtail butterfly,Papilio xuthus L. (Lepidoptera: Papilionidae). Appl Ent Zool 17:151–159
Wiklund C (1975) The evolutionary relationship between oviposition preferences and larval host range inPapilio machaon L. Oecologia 18:185–197
Wiklund C (1981) Generalist vs. specialist oviposition behaviour inPapilio machaon (Lepidoptera) and functional aspects on the hierarchy of oviposition preferences. Oikos 36:163–170
Wiklund C (1982) Generalist vs. specialist utilization of host plants among butterflies. In: Visser JH, Minks AK (eds) Insect-plant relationships. Pudoc, Wageningen, Netherlands, pp 181–191
Winer BJ (1962) Statistical principles in experimental design. McGraw-Hill, NY
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scriber, J.M., Giebink, B.L. & Snider, D. Reciprocal latitudinal clines in oviposition behavior ofPapilio glaucus andP. canadensis across the Great Lakes hybrid zone: possible sex-linkage of oviposition preferences. Oecologia 87, 360–368 (1991). https://doi.org/10.1007/BF00634592
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00634592