Abstract
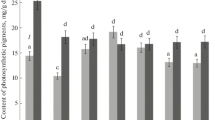
Mature green leaves from tobacco (Nicotiana tabacum L.) plants were submitted to contrasting light conditions; half of each leaf was shaded (changed from 60 to 25 μmol photons· m-2 ·s-1=LL) and the other half was exposed to higher light (changed from 60 to 360 μmol·m-2· s-1=HL) for 24 h. The activity and quantity of ribulose-1,5-bisphosphate carboxylase (RuBPCase) were measured during the first 24 h in each leaf region and the variation was compared with that of small subunit (SSU)-and large subunit (LSU)-mRNA contents determined by a hybridot technique. Each leaf half responded separately to the actual light received. The activity of RuBPCase increased progressively in the HL zones and decreased in the LL zones. The RuBPCase-protein content was not significantly modified during the first 24 h but SSU-mRNA content responded very rapidly to the treatment. Within 2 h a significant difference in SSU mRNA appeared between LL and HL zones: at the end of the photoperiod the content in LL zones was approx. 25% of the initial value. The increase in the exposed zone, however, was not significant, indicating that there was a dissymmetry of the response to variation in incident white light. The LSU-mRNA contents from the same leaf extracts were totally unaffected by the light treatment. No day-night variations were noted in either SSU or LSU mRNAs in control plants.
Similar content being viewed by others
Abbreviations
- HL:
-
high-light irradiance
- LL:
-
lower-ligh irradiance
- LSU:
-
large subunit of RuBPCase
- RuBPCase:
-
ribulose-1,5-bisphosphate carboxylase
- SSU:
-
small subunit of RuBPCase
References
Abbott, M.S., Bogorad, L. (1987) Light regulation of genes for the large and small subunits of ribulose-bisphosphate carboxylase in tobacco. In: Progress in photosynthesis research vol. IV-9, pp. 527–534, Biggins, J. ed. Martinus Nijhoff, Dordrecht
Berry, J.O., Nikolau, B.J., Carr, J.P., Klessig, D.F. (1985) Transcriptional and post-transcriptional regulation of ribulose-1,5-bisphosphate carboxylase gene expression in light and dark-grownAmaranthus cotyledons. Mol. Cell. Biol.5, 2238–2246
Berry, J.O., Nikolau, B.J., Carr, J.P., Klessig, D.F. (1986) Translation regulation of light-induced ribulose-1,5-bisphosphate carboxylase gene expression in Amaranth. Mol. Cell. Biol.6, 2347–2353
Berry-Lowe, S.L., Meagher, R.B. (1985) Transcriptional regulation of a gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean tissue is linked to the phytochrome response. Mol. Cell. Biol.5, 1910–1917
Boardman, N.K. (1977) Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol.28, 355–377
Gallagher, T.F., Ellis, R.J. (1982) Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J.1, 1493–1498
Gallagher, T.F., Jenkins, G.I., Ellis, R.J. (1985) Rapid modulation of transcription of nuclear genes encoding chloroplast proteins by light. FEBS Lett.186, 241–245
Inamine, G., Nash, B., Weissbach, H., Brot, N. (1985) Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: evidence for translational control. Proc. Natl. Acad. Sci. USA82, 5690–5694
Kloppstech, K. (1985) Diurnal and circadian rhythmicity in the expression of light-induced plant nuclear messenger RNAs. Planta165, 502–506
Morelli, G., Nagy, F., Fraley, R.T., Rogers, S.G., Chua, N.H. (1985) A short conserved sequence is involved in the light-inducibility of a gene encoding ribulose-1,5-bisphosphate carboxylase small subunit of pea. Nature31, 200–204
Nelson, T., Harpster, M.H., Mayfield, S.P., Taylor, W.C. (1984) Light-regulated gene expression during maize leaf development. J. Cell Biol.98, 558–564
Perchorowicz, J.T., Raynes, D.A., Jensen, R.G. (1981) Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc. Natl. Acad. Sci. USA78, 2985–2989
Prioul, J.L., Brangeon, J., Reyss, A. (1980a) Interaction between external and internal conditions in the development of the photosynthetic features in a grass leaf. I. Regional responses along a leaf during and after low-light or high-light acclimation. Plant Physiol.66, 762–769
Prioul, J.L., Brangeon, J., Reyss, A. (1980b) Interaction between external and internal conditions in the development of the photosynthetic features in a grass leaf. II. Reversibility of light induced responses as a function of developmental stages. Plant Physiol.66, 770–774
Prioul, J.L., Reyss, A. (1987) Acclimation of ribulose-bisphosphate carboxylase and mRNAs to changing irradiance in adult tobacco leaves. Differential expression in LSU and SSU mRNA. Plant Physiol.84, 1238–1243
Reyss, A., Prioul, J.L. (1975) Carbonic Anhydrase and carboxylases activities from plants (Lolium multiflorum) adapted to different light regimes. Plant Sci. Lett.5, 189–195
Salvucci, M.E., Portis, A.R. Jr., Ogren, W.L. (1985) A soluble chloroplast protein catalyzes activation of ribulose bisphosphate carboxylase/oxygenase in vivo. Photosynth. Res.7, 193–201
Salvucci, M.E., Portis, A.R. Jr., Ogren, W.L. (1986) Light and CO2 response of ribulose-1,5-bisphosphate carboxylase/oxygenase activation inArabidopsis leaves. Plant Physiol.80, 655–659
Sasaki, Y., Tomoda, Y., Kamikubo, T. (1984) Light regulates the gene expression of ribulose bisphosphate carboxylase at the levels of transcription and gene dosage in greening pea leaves. FEBS Lett.173, 31–35
Sebaa, El.D., Prioul, J.L., Brangeon, J. (1987) Acclimation of adultLolium multiflorum leaves to change in irradiance: effect on leaf photosynthesis and chloroplast ultrastructure. J. Plant Physiol.127, 431–441
Seemann, J.R., Berry, J.A., Freas, S.M., Krump, M.A. (1985) Regulation of ribulose bisphosphate carboxylae activity in vivo by a light-modulated inhibitor of catalysis. Proc. Natl. Acad. Sci. USA82, 8024–8028
Servaites, J.C. (1985) Binding of a phosphorylated inhibitor to ribulose bisphosphate carboxylase/oxygenase during the night. Plant Physiol.78, 839–843
Shinozaki, K., Sugiura, M. (1982) The nucleotide sequence of tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene20, 91–102
Silverthorne, J., Tobin, E.M. (1984) Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc. Natl. Acad. Sci. USA81, 1112–1116
Vernet, T., Fleck, J., Durr, A., Fritsch, C., Pinck, M., Hirth, L. (1982) Expression of the gene coding for the small subunit of ribulose bisphosphate carboxylase during differentiation of tobacco plant protoplasts. Eur. J. Biochem.126, 489–494
Walden, R., Leaver, C.J. (1981) Synthesis of chloroplast proteins during germination and early development of cucumber. Plant Physiol.67, 1090–1096
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prioul, JL., Reyss, A. Rapid variations in the content of the RNA of the small subunit of ribulose-1,5-bisphosphate carboxylase of mature tobacco leaves in response to localized changes in light quantity. Relationships between the activity and quantity of the enzyme. Planta 174, 488–494 (1988). https://doi.org/10.1007/BF00634477
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00634477