Summary
The effects of long-term incubation and of indomethacin on contractile responses to adrenaline and noradrenaline of the guinea-pig esophageal muscularis mucosae were studied in vitro.
In the presence of propranolol (3 μM), contractions of the isolated muscularis mucosae of the guinea-pig esophagus induced by adrenaline (10 μM) and noradrenaline (10 μM) gradually increased in magnitude with incubation time, and after 7.5 h reached a peak which was about 3 times the initial response. Contractions to adrenaline and noradrenaline observed at 2.5 h after the incubation was started were fully inhibited by phentolamine (3 μM) and prazosin (3 μM), and slightly by yohimbine (3 μM). These responses were also inhibited by polyphloretin phosphate (PPP, 30 μg/ml), indomethacin (1 μM) and aspirin (100 μM). The augmented contractions to adrenaline and noradrenaline observed at 7.5 h after the incubation was started were also inhibited by PPP, indomethacin and aspirin, while they were rather resistant to the blocking actions of phentolamine, prazosin and yohimbine.
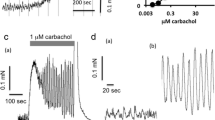
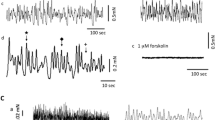
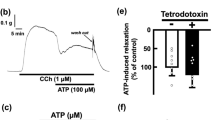
The effect of matched concentrations of carbachol (0.1 μM), prostaglandin E2 (PGE2, 0.01 μM) and arachidonic acid (0.2 μM), which produced the same degree of contraction induced by catecholamines (10 μM), was gradually increased with incubation time. When the strip was treated with indomethacin (1 μM) throughout the experiment, contraction to arachidonic acid was abolished, that to carbachol was almost constant during long-term incubation but that to PGE2 was still increased after long-term incubation. Moreover, both concentration-response curves to carbachol and PGE2 examined at 7.5 h after the incubation was started were located to the left to those examined at 1.5 h after the incubation was started. In the presence of indomethacin (1 μM), the curve to PGE2, but not to carbachol, still shifted to the left after long-term incubation.
The present results demonstrated that the contractile responses of the guinea-pig esophageal muscularis mucosae were increased in magnitude depending on the incubation time. The mechanism of this augmentation may involve the development of atypical adrenoceptors, the stimulated arachidonic acid cascade and the increased responsiveness to PGs.
Similar content being viewed by others
References
Bailey DM (1965) The action of sympathomimetic amines on circular and longitudinal smooth muscle from the isolated oesophagus of the guinea-pig. J Pharm Pharmacol 17:782–787
Bailey DM (1971) Inhibitory and excitatory effects of sympathomimetic amines on muscle strips from the stomach of the guinea-pig. Br J Pharmacol 41:227–238
Bauer V (1982) Distribution and types of adrenoceptors in the guinea-pig ileum: the action of α-and β-adrenoceptors blocking agents. Br J Pharmacol 76:569–578
Bennett A, Posner J (1971) Studies on prostaglandin antagonists. Br J Pharmacol 42:584–594
Bennett A, Eley KG, Stockley HL (1975) The effects of prostaglandins on guinea-pig isolated intestine and their possible contribution to muscle activity and tone. Br J Pharmacol 54:197–204
Bennett A, Stamford IF, Stockley HL (1977) Estimation and characterization of prostaglandins in the human gastrointestinal tract. Br J Pharmacol 61:579–586
Borda E, Agostini MDC, Sterin-Borda L, Gimeno MF, Gimeno AL (1981) Inhibitory effects of some catecholamines on contractions of uterine strips isolated from estrus and spayed rats. Influence of endogenous and exogenous prostaglandins on the action of methoxamine. Eur J Pharmacol 69:55–62
Botting JH, Salzmann R (1974) The effect of indomethacin on the release of prostaglandin E2 and acetylcholine from guinea-pig isolated ileum at rest and during field stimulation. Br J Pharmacol 50:119–124
Brückner-Schmidt R, Jackisch R, Hertting G (1981a) Stimulation of prostaglandin E2-synthesis by noradrenaline in primary cell cultures from rabbit splenic pulpa is mediated by atypical α-adrenoceptors. Naunyn-Schmiedeberg's Arch Pharmacol 316:1–7
Brückner-Schmidt R, Jackisch R, Hertting G (1981b) On the mechanism of noradrenaline-induced prostaglandin E2 synthesis in primary cell cultures from rabbit splenic pulpa. Arch Int Pharmacodyn Ther 253:266–277
Bülbring E, Ohashi H, Tomita T (1981) Adrenergic mechanisms. In: Bülbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle: an assessment of current knowledge. Edward Arnold Publishers, London, pp 219–248
Christensen J, Daniel EE (1966) Electric and motor effects of autonomic drugs on longitudinal esophageal smooth muscle. Am J Physiol 211:387–394
Coburn RF (1980) Evidence for oscillation of prostaglandin release from spontaneously contracting guinea-pig taenia coli. Am J Physiol 239:G53-G58
Coupar IM, McLennan PL (1978) The influence of prostaglandins on noradrenaline-induced vasoconstriction in isolated perfused mesenteric blood vessels of the rat. Br J Pharmacol 62:51–59
Doggrell SA, Scott GW (1980) The effects of time and indomethacin on contractile responses of the guinea-pig gall bladder in vitro. Br J Pharmacol 71:429–434
Frankhuijzen AL, Bonta IL (1975) Role of prostaglandins in tone and effector reactivity of the isolated rat stomach preparation. Eur J Pharmacol 31:44–52
Kamikawa Y, Shimo Y (1979a) Cholinergic and adrenergic innervations of the muscularis mucosae in guinea-pig esophagus. Arch Int Pharmacodyn Ther 238:220–232
Kamikawa Y, Shimo Y (1979b) Antagonistic effect of dipyridamole on the prostaglandin F2α-induced contraction of the guinea-pig esophageal smooth muscle. Dokkyo J Med Sci 6:52–58
Kamikawa Y, Serizawa K, Shimo Y (1977) Some possibilities for prostaglandin mediation in the contractile response to ATP of the guinea-pig digestive tract. Eur J Pharmacol 45:199–203
Kamikawa Y, Shimo Y, Uchida K (1982) Inhibitory actions of catecholamines on electrically induced contractions of the submucous plexus-longitudinal muscularis mucosae preparation of the guineapig oesophagus. Br J Pharmacol 76:271–277
King CE, Robinson MH (1945) The nervous mechanisms of the muscularis mucosae. Am J Physiol 143:325–335
Lee CY (1970) Adrenergic receptors in the intestine. In: Bülbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle. Edward Arnold Publishers, London, pp 549–557
Ligumsky M, Grossman MI, Kauffman Jr GL (1982) Endogenous gastric mucosal prostaglandins: their role in muscosal integrity. Am J Physiol 242:G337-G341
McGrath JC (1982) Evidence for more than one type of postjunctional α-adrenoceptor. Biochem Pharmacol 31:467–484
Minneman KP, Pittman RN, Molinoff PB (1981) β-Adrenergic receptor subtypes: properties, distribution, and regulation. Ann Rev Neurosci 4:419–461
Munro AF (1953) Effect of autonomic drugs on the responses of isolated preparations from the guinea-pig intestine to electrical stimulation. J Physiol 120:41–52
Olson RD, Nies AS, Gerber JG (1981) Alpha adrenergic-mediated renin release is prostaglandin-dependent J Pharm Exp Ther 219:321–325
Pipili E, Poyser NL (1981) Effects of nerve stimulation and of administration of noradrenaline or potassium chloride upon the release of prostaglandin I2, E2 and F2α from the perfused mesenteric arterial bed of the rabbit. Br J Pharmacol 72:89–93
Sanders KM, Ross G (1978) Effects of endogenous prostaglandin E on intestinal motility. Am J Physiol 234:E204-E208
Starke K (1981) α-Adrenoceptor subclassification. Rev Physiol Biochem Pharmacol 88:199–236
Tepperman BL, Soper BD (1981) Distribution of prostaglandin E2 binding sites in the porcine gastrointestinal tract. Prostaglandins 22:205–212
Uchida K (1983) Pharmacological characterization of the adrenergic receptors in the muscularis mucosae of the guinea-pig esophagus. Fol Pharmacol Jap 82: (in press)
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action of aspirin-like drugs. Nature New Biol 231:232–235
Walder DN (1953) The muscularis mucosae of the human stomach. J Physiol 120:365–372
Wennmalm Å, Brundin T (1978) Prostaglandin-mediated inhibition of noradrenaline release: IV. Prostaglandin synthesis is stimulated by myocardial adrenoceptors differing from the α-and β-type. Acta Physiol Scand 102:374–381
Whittle BJR (1981) Temporal relationship between cyclooxygenase inhibition, as measured by prostaglandin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterol 80:94–98
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uchida, K., Kamikawa, Y. & Shimo, Y. Time-dependent augmentation of the contractile responses to adrenaline and noradrenaline of the guinea-pig esophageal muscularis mucosae in vitro. Naunyn-Schmiedeberg's Arch. Pharmacol. 323, 114–120 (1983). https://doi.org/10.1007/BF00634258
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00634258