Abstract
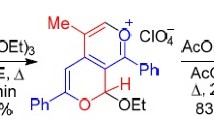
When 2,6-diaryl-4-phenylethynylpyrylium perchlorates are refluxed in water, methanol, or ethanol, they are converted to monomethinecyanines, the formation of which is explained by hydration of the phenylethynyl group and subsequent [2+2]-cycloaddition of the resulting 2,6-diaryl-4-benzoylmethylenepyrans to the starting pyrylium salt. The corresponding pyridine derivatives were obtained by the action of ammonia and aniline on the monomethinecyanines. The IR and PMR spectra of the compounds and the results of x-ray diffraction analysis of 2,6-diphenyl-4-[1′-(2″,6″-diphenyl-4″-pyranylidene)-2′-phenyl-3′-benzoylallyl]pyridine are presented.
Similar content being viewed by others
Literature Cited
H. H. Wiehe (editor), The Chemistry of Acetylenic Compounds [Russian translation], Khimiya, Moscow (1973).
T. T. Tidwell, Angew. Chem.,96, 16 (1984).
H. Mayr and E. BÄuml, Tetrahedron Lett.,25, 1127 (1984).
H. Perst, Oxonium Ions in Organic Chemistry, Verlag Chemie, AP, New York-London (1971).
G. N. Dorofeenko, A. V. Koblik, L. A. Murad'yan, T. I. Polyakova, and B. A. Tertov, Zh. Org. Khim.,16, 1741 (1980).
A. V. Koblik, L. A. Murad'yan, S. G. Blagorodov, L. N. Chernavskaya, and N. A. Dmitrieva, USSR Author's Certificate No. 1,189,861; Byull. Izobret., No. 41, 103 (1985).
A. T. Balaban, A. Dinculescu, G. N. Dorofeenko, G. W. Fischer, A. V. Koblik, V. V. Mezheritskii and W. Schroth, Advances in Heterocyclic Chemistry, Supplement 2, Academic Press, New York (1982), p. 68.
A. I. Pyshchev, N. G. Bokii, and Y. T. Struchkov, Tetrahedron,34, 2131 (1978).
A. I. Kitaigorodskii, M. P. Zorkii and V. K. Bel'skii, The Structures of Organic Substances [in Russian], Nauka, Moscow (1980).
D. Chasseau, J. Gaultier, C. Hauw, R. Fugnitto, V., Gianis, and H. Srtzelecka, Acta Cryst.,B38, 1629 (1982).
Tables of Interatomic Distances and Configurations in Molecules and Ions, Special Publication No. 18, Chem. Soc., London (1965).
A. I. Kitaigorodskii (Kitaigorodsky), Molecular Crystals and Molecules, Academic Press, New York-London (1973).
R. G. Gerr, A. I. Yanovskii, and Yu. T. Struchkov, Kristallografiya,28, 1029 (1983).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 885–890, July, 1988.
Rights and permissions
About this article
Cite this article
Koblik, A.V., Murad'yan, L.A., Kompan, O.E. et al. Ethynyl carbonium ions. 1. Reaction of 2.6-diaryl-4-phenylethynylpyrulium perchlorates with oxygen-containing nucleophiles. Chem Heterocycl Compd 24, 725–730 (1988). https://doi.org/10.1007/BF00633162
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00633162