Abstract
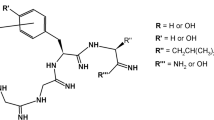
The synthesis has been effected of the amide of the tetrapeptide forming the sequence 6–9 of oxytocin with the use of benzyl protection of the thiol function of cysteine by two main schemes 1+3 and 2+2. The advantageousness of performing the synthesis by the 2+2 scheme has been shown. The overall yield of tetrapeptide using the method of condensation with the formation of mixed anhydrides amounted to 51% by the scheme proposed.
Similar content being viewed by others
Literature cited
Zh. D. Bespalova et al., Khim. Prir. Soedin., 808 (1973); Zh. D. Bespalova et al., Zh. Obshch. Khim.,44, No. 1 (1973).
S. Ya. Miksta et al., Izv. Akad. Nauk, LatvSSR, Ser. Khim., No. 5, 618–621 (1975).
Additional information
All-Union Scientific-Research Institute of the Technology of Blood Substitutes and Hormone Preparations, Moscow. Translated from Khimiya Prirodnykh Soedinenii, Nos. 3,4, pp. 393–400, May–August, 1992.
Rights and permissions
About this article
Cite this article
Ivanov, A.K., Antonov, A.A. & Donetskii, I.A. Synthesis of the amide of the C-terminal tetrapeptide of the sequence of oxytocin. Chem Nat Compd 28, 344–349 (1992). https://doi.org/10.1007/BF00630256
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00630256