Abstract
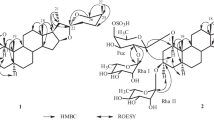
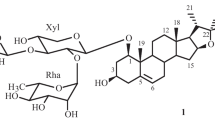
A glycoside has been isolated from the plantNolina microcarpa S. Wats. (family Dracaenaceae) which has been called nolinofuroside B and has the structure of 6β-methoxy-3β,5-cyclo-(25S)-furostan-1β,22α,26-triol 1-O-β-D-fucopyranoside 26-O-β-D-glucopyranoside (I). Enzymatic hydrolysis of the bioside (I) yielded nolinospiroside B (III) and nolinogenin (IV). The latter is 6β-methoxy-3β,5-cyclo-(25S)-spirostan-6β-ol, and glycoside (III) is its 1-O-β-D-fucopyranoside.
Similar content being viewed by others
Literature cited
G. B. Shevchuk, Yu. S. Vollerner, A. S. Shashkov, and V. Ya. Chirva, Khim. Prir. Soedin., 672 and 678 (1991).
G. B. Shevchuk, Yu. S. Vollerner, A. S. Shashkov, M. B. Gorovits, and V. Ya. Chirva, Khim. Prir. Soedin., 801 (1991).
A. V. Kamernitskii, N. K. Abubakirov, M. B. Gorovits, Yu. S. Vollerner, N. E. Voishvillo, I. G. Reshetova, and V. A. Paseshnichenko, The Chemistry of the Spirostanols [in Russian], Moscow (1986), pp. 8–37.
E. Stahl, Thin Layer Chromatography, 1st English edition, Springer/Academic Press, New York (1965).
R. Kasai, M. Okihara, I. Asakawa, K. Mizutani, and K. Tanaka, Tetrahedron,35, 1427 (1979).
C. Altona and C. A. G. Hasnoot, Org. Magn. Res.,13, No. 6, 417 (1980).
S. Seo, Y. Tomita, K. Tori, and Y. Yoshimura, J. Am. Chem. Soc.,100, No. 11, 3331 (1978).
M. E. Wall, C. R. Eddy, M. L. McClennan, and M. E. Klumpp, Anal. Chem.,24, 1337 (1952).
C. R. Eddy, M. E. Wall, and M. K. Scott, Anal. Chem.,25, 266 (1953).
W. H. Faul and C. Djerassi, Org. Mass Spectrom.,3, 1187 (1970).
M. I. Isaev, M. B. Gorovits, and N. K. Abubakirov, Khim. Prir. Soedin., 156 (1989).
M. S. Gonzalez, D. A. Bustos, M. Zudenigo, and E. A. Ruveda, Tetrahedron,42, 755 (1986).
T. T. Gorovits, Khim. Prir. Soedin., 263 (1970).
Additional information
M. V. Frunze Simferopol' State University. Institute of Chemistry of Plant Substances, Uzbekistan Academy of Sciences, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 218–224, March–April, 1992.
Rights and permissions
About this article
Cite this article
Shevchuk, G.V., Vollerner, Y.S., Shashkov, A.S. et al. Steroids of the spirostan and furostan series from Nolina microcarpa IV. Structures of nolinogenin, nolinospiroside B, and nolinofuroside B. Chem Nat Compd 28, 187–192 (1992). https://doi.org/10.1007/BF00630172
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00630172