Summary
A series of cobalt(II) and nickel(II) 2-X-1,3,4-thiadiazole complexes (X=Cl or Br) were investigated by electronic, n.m.r., Raman and i.r. spectroscopy, and by thermogravimetric analyses, magnetic moments and conductivity measurements.
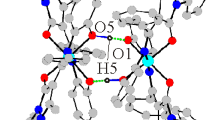
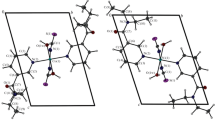
The complexes havepseudo-tetrahedral or pseudo-octa-hedral stereochemistries with MN2X2 (X=Cl or Br), MN4X2 (X=Cl, Br or O) or MN3O3 chromophores.
The ligands are mono- or bi-dentate without involving the sulphur atom. Surprisingly, the chloro-derivative ligand seems to be more nucleophilic than the bromo-analogue.
The computed (CNDO/2) molecular orbital indices for the uncomplexed ligands indicate that the coordination sites are the nitrogen atoms.
In connection with our previous work(1) on the coordination ability of heterocyclic ligands with interesting biological(2,3) and pharmaceutical(4) properties and containing endocyclic nitrogen and sulphur donor atoms, we have considered the 1,3,4-thiadiazole ring, which is the main framework of a very important class of diuretic drugs, the sulphonamide inhibitors(5) of the zinc m tallo-enzyme carbonic anhydrase.
In this study we have investigated the coordination behaviour of the 2-chloro-1,3,4-thiadiazole(ctz) and the 2-bromo-1,3,4-thiadiazole (btz).
Similar content being viewed by others
References
A. C. Fabretti and G. C. Franchini,Transition Met. Chem., 9, 190 (1984).
S. Lindskog,Advances in Inorganic Biochemistry; vol. 4, Elsevier Biomedical, p. 115, 1982.
I. Bertini and C. Luchinat,Acc. Chem. Res., 16, 272 (1983).
R. C. Allen, in E. J. Cragoe Jr. (Ed.),Sulfonamide Diuretics, in Diuretics Chemistry, Pharmacology,and Medicine, Wiley, p. 49, 1983.
P. G. De Benedetti, C. Frassineti and M. C. Menziani,Quant. Struct.-Act. Relat., in press.
W. J. Geary,Coord. Chem. Rev., 7, 81 (1971).
K. Nakamoto,Infrared Spectra of Inorganic and Coordination Compounds, pp. 155–158, Wiley, New York, 1970.
N. B. Colthup, L. H. Daly and S. E. Wiberley,Introduction to Infrared and Raman spectroscopy, Academic Press, New York, London, 1964.
P. Mirone,Gazz. Chim. Ital., 86, 165 (1956).
B. Bok, S. Brodersen and L. Hansen,Acta Chem. Scand., 9, 749 (1955).
J. A. Pople and G. A. Segal,J. Chem. Phys., 44, 3289 (1966).
R. E. Brown and A. M. Simas,Theoret. Chim. Acta, 62, 1 (1982).
K. Fukui,Angew. Chem., 21, 801 (1982).
A. B. P. Lever,Inorganic Electronic Spectroscopy, Elsevier, 1968.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benedetti, A., De Benedetti, P.G., Fabretti, A.C. et al. Coordination behaviour of 2-halo-1,3,4-thiadiazoles towards cobalt(ii) and nickel(ii). Transition Met Chem 9, 457–460 (1984). https://doi.org/10.1007/BF00620677
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00620677