Summary
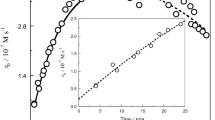
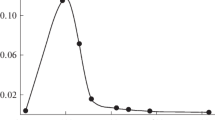
A cherry-red transitory coloration is formed when OsO4 or osmium(VI) is added to alkaline hydrogen peroxide solution in the pH range 9–13. The transient has an absorption maximum at 530 nm and its concentration depends on the pH of reaction mixture reaching a maximum at pH 10.5–11. The transient is designated as a peroxo-derivative of osmium(VIII) [or an osmium(VII) — Superoxide radical pair if the peroxoderivative undergoes a fast intramolecular one-electron transfer].
Many decades ago it was observed1 that a cherry-red coloration appears transiently when osmium tetroxide solution is added to alkaline hydrogen peroxide solution. However, to our knowledge, there are no literature data about the nature of this transient. Further, we observed recently that there is a close connection between the rate of decomposition of hydrogen peroxide catalysed by osmium tetroxide and the intensity of the coloration, and therefore an attempt was made to investigate the transient by using a fast spectrophotometer technique.
Similar content being viewed by others
References
L. Chugaev, N. Bikermann,Z. Anorg. Allg. Chem., 172, 229 (1928).
N. Marek, L. Szekeres,Spectrophotometer for following Fast Reactions, Hungarian Patent 165394 (1972).
W. P. Griffith,The Chemistry of the Rarer Platinium Metals, Intersci. Publs. London 1967 p. 64.
Z. M. Galbács, Á. Zsednai, L. J. Csányi,Transition Met. Chem., 8, 328 (1983).
R. D. Sauerbrunn, E. B. Sandell,J. Am. Chem. Soc., 75, 4170 (1953).
S. C. Bavay, G. Nowogrocky, G. Tridot,Bull. Soc. Chim. Fr., 6, 2030 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Csányi, L.J., Galbács, Z.M., Nagy, L. et al. Nature of the cherry-red transient formed during the interaction of osmium tetroxide with hydrogen peroxide. Transition Met Chem 11, 319–320 (1986). https://doi.org/10.1007/BF00620656
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00620656