Summary
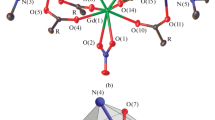
A change of the reported medium of reaction oftrans- [CrCl2(OH2)4]Cl · 2H2O witho-R2AsC6H4CO2M (M = Na), from 95% ethanol to acetone, results in the change of an hydroxo — to an oxo — group (R = Ph), in the number of water molecules (R = Et or C6H11) and/or of ligand molecules (R =p-tolyl) in chromium(III)-arsine complexes. However, for R = Me, the same complex is obtained in each case. I.r. spectral data of these complexes favour the chelation of the carboxylate ion and non-coordination of arsenic(III) except for CrO(o-Ph2AsC6H4CO2) · 2.5 H2O in which arsenic(III) of the monotertiary arsine group appears to coordinate to chromium(III). This would seem to be the first example of this type. On the other hand, the reaction of CrO3 witho-R2As- C6H4CO2M (M = H) in 1∶4.5 molar ratio in acetone yields only one type of complex,viz., [Cr3O3(o-R2As(O)C6H4-CO2)2(o-R2AsC6H4CO2)(H2O)6] · n H2O (n = 2, R = Me, C6H11 or Ph; n = O, R = Et). The arsine oxide molecules appear to chelate through As = 0 and the carboxylate oxygens while the arsine ligand binds only through the carboxylate oxygens leaving arsenic(III) uncoordinated as reported for the complexes obtained from the same reactants in 95% ethanol.
Similar content being viewed by others
References
C. A. McAuliffe,Transition Metal Complexes of Phosphorus, Arsenic and Antimony Ligands, Macmillan 1973; N. V. Maatschappij, G. C. Lawrence, H. S. Lynn and D. M. Richard,Ger. Pat. 1, 186455 (1965). C.A. 62; 16054f; R. S. Nyholm and G. J. Sutton,J. Chem. Soc., 560 (1958); K. Gustav and H. Moerke,Z. Chemie, 9, 32 (1969); R. O. Feltham and W. Silverthorn,Inorg. Chem., 7, 1154 (1968); J. Lewis, R. S. Nyholm, C. S. Pande, S. S. Sandhu and M. H. B. Stiddard,J. Chem. Soc., 3009 (1964), R. J. H. Clark, M. L. Greenfield and R. S. Nyholm,J. Chem. Soc. A, 1254 (1966).
L. R. Gray, A. L. Hale, W. Levason, F. P. McCullough and M. Webster,J. Chem. Soc., Dalton Trans., 47 (1984).
A. Tzchach and H. Nindel,J. Organometal. Chem., 24, 159 (1970).
S. S. Parmar and H. Kaur,Transition Met. Chem., 7, 79 (1982).
G. N. Reilly, R. W. Schmid and F. S. Sadek,J. Chem. Educ., 36, 555 (1959).
S. S. Parmar, H. K. Saluja and H. K. Bathla, unpublished work.
W. R. Cullen and P. S. Dhaliwal,Can. J. Chem., 45, 719 (1967).
F. F. Blicke and L. D. Powers,J. Am. Chem. Soc., 54, 3353 (1932).
D. M. Adams,Metal Ligand and Related Vibrations, Arnold, London, 1967, p. 238.
K. Nakamoto,Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York (1978).
J. Fujika, K. Nakamoto and M. Kobayashi,J. Am. Chem. Soc., 78, 3963 (1956).
M. D. Taylor, C. P. Carter and C. I. Wynter,J. Inorg. Nucl. Chem., 30, 1503 (1968); T. J. Delia, M. A. Little and D. X. West,J. Inorg. Nucl. Chem., 35, 1400 (1973).
S. S. Parmar and H. K. Bathala,Indian J. Chem., (1985), in press.
S. S. Parmar and H. Kaur,Polyhedron, 1, 667 (1982).
S. S. Parmar, H. K. Bharj and M. L. Sehgal,Polyhedron, 4, 959 (1985).
D. J. Hodgson,Prog. Inorg. Chem., 19, 173 (1975).
A. Earnshaw and J. Lewis,J. Chem. Soc., 396 (1961).
C. A. McAuliffe and W. D. Perry,Inorg. Nucl. Chem. Lett., 10, 367 (1974).
T. Morishita, K. Hori, E. Kyuno and R. Tschida,J. Bull. Chem. Soc., 38, 1276 (1965).
D. J. Hewkin and W. P. Griffith,J. Chem. Soc. A, 472 (1966) and refs. therein.
E. Konig,Structure and Bonding, 9, 175 (1971).
L. Dubichi and R. L. Martin,Aust. J. Chem., 23, 215 (1970).
M. Ciampolini,Structure and Bonding, 6, 52 (1969).
F. H. Westheimer,Chem. Rev., 45, 419 (1949).
K. B. Wiberg,Oxidation by Chromic acid and Chromyl Compounds', in Oxidation inOrganic Chemistry, Part 4, Acadamic Press, New York (1965).
J. G. Mason and A. D. Rowalak,Inorg. Chem., 3, 1248 (1964).
A. Haim,Prog. Inorg. Chem., 30, 273 (1983).
I. M. Kolthoff and M. A. Fineman,J. Phys. Chem., 60, 1383 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parmar, S.S., Bharaj, H.K. & Saighal, M.L. Metal complexes of hybrid oxygen-arsenic ligands. Part V. A study of the products fromo-R2AsC6H4CO2M and CrO3 (M = H)/trans-[CrCl2(OH2)4]Cl · 2H2O (M = Na) in acetone. Transition Met Chem 11, 283–286 (1986). https://doi.org/10.1007/BF00620646
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00620646