Summary
-
1.
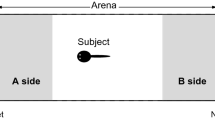
Larvae of the American toad (Bufo americanus) preferentially associate with their siblings in laboratory tests, suggesting that they can recognize kin. The sensory basis of their kin recognition abilities was investigated by measuring the responses of individuals in a Y-maze to waterborne cues emanating from their siblings and from nonsiblings.
-
2.
When simultaneously presented with water flowing from two containers, each holding members of a different sibling group, test subjects spent significantly more time oriented toward their siblings than toward non-siblings. Similar results were obtained when the stimulus water was first passed through intermediary reservoirs. Hence, kinship cues are unlikely to be acoustic or vibratory stimuli perceived by the auditory or lateral line systems.
-
3.
Tadpoles whose external nares were blocked with a gelatinous paste did not behaviorally discriminate between water flowing from siblings and that flowing from non-siblings. Retested after their nares were unplugged, these individuals oriented significantly toward their siblings, as did shamtreated test individuals.
-
4.
Stimulus water conditioned by sibling groups and then stored for 24–30 h failed to elicit a discrimination response, indicating that kinship cues released by tadpoles lose their effectiveness during this period. Signals with rapid fade-out times would probably be more efficient under natural conditions than those that persisted after individuals had moved.
-
5.
Test subjects simultaneously presented with water flowing from siblings and blank (dechlorinated tap) water showed no tendencies to discriminate between these stimuli. When individuals were exposed both to water from non-siblings and to blank water, however, they oriented significantly toward the blank water. Kin association may thus result in part from negative klinokinetic responses induced by contact with factors released by nonkin.
-
6.
Chemical cues released by tadpoles appear sufficient to communicate their kinship identity. These cues are probably perceived and processed by the main olfactory system. Kin recognition mechanisms may be further elucidated by chemical and neurophysiological analyses.
Similar content being viewed by others
References
Alexander RD (1979) Darwinism and human affairs. University of Washington Press, Seattle
Bateson P (1982) Preferences for cousins in Japanese quail. Nature 295:236–237
Beecher IM, Beecher MD (1983) Sibling recognition in bank swallows (Riparia riparid). Z Tierpsychol 62:145–150
Beiswenger RE (1977) Diel patterns of aggregative behavior in tadpoles ofBufo americanus, in relation to light and temperature. Ecology 58:98–108
Blaustein AR, O'Hara RK (1981) Genetic control for sibling recognition? Nature 290:246–248
Blaustein AR, O'Hara RK (1982a) Kin recognition cues inRana cascadae tadpoles. Behav Neural Biol 36:77–87
Blaustein AR, O'Hara RK (1982b) Kin recognition inRana cascadae tadpoles: maternal and paternal effects. Anim Behav 30:1151–1157
Blaustein AR, O'Hara RK (1983) Kin recognition inRana cascadae tadpoles: effects of rearing with nonsiblings and varying the strength of the stimulus cues. Behav Neural Biol 39:259–267
Buckle GR, Greenberg L (1981) Nestmate recognition in sweat bees (Lasioglossum zephyrum): does an individual recognize its own odour or only odours of its nestmates? Anim Behav 29:802–809
Colgan PW (1983) Comparative social recognition. Wiley, New York
Costa HH (1967) Avoidance of anoxic water by tadpoles ofRana temporaria. Hydrobiologia 30:374–384
Døving KB, Nordeng H, Oakley B (1974) Single unit discrimination of fish odours released by char (Salmo alpinus L.) populations. Comp Biochem Physiol 47A:1051–1063
D'Udine B, Partridge L (1981) Olfactory preferences of inbred mice (Mus musculus) for their own strain and for siblings: effects of strain, sex and cross-fostering. Behaviour 78:314–324
Fisknes B, Døving KB (1982) Olfactory sensitivity to groupspecific substances in Atlantic salmon (Salmo salar L.). J Chem Ecol 8:1083–1092
Foster MS, McDiarmid RW (1982) Study of aggregative behavior ofRhinophrynus dorsalis tadpoles: design and analysis. Herpetologica 38:395–404
Fredrickson WT, Sackett GP (1984) Kin preferences in primates (Macaca nemestrina): relatedness or familiarity? J Comp Psychol 98:29–34
Gilder PM, Slater PJB (1978) Interest of mice in conspecific male odours is influenced by degree of kinship. Nature 274:364–365
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Greenberg L (1979) Genetic component of bee odor in kin recognition. Science 206:1095–1097
Hamilton WD (1964) The genetical evolution of social behaviour. I, II. J Theor Biol 7:1–52
Holmes WG (1984) Sibling recognition in thirteen-lined ground squirrels: effects of genetic relatedness, rearing association, and olfaction. Behav Ecol Sociobiol 14:225–233
Holmes WG, Sherman PW (1982) The ontogeny of kin recognition in two species of ground squirrels. Am Zool 22:491–517
Holmes WG, Sherman PW (1983) Kin recognition in animals. Am Scient 71:46–55
Jaeger RG (1981) Dear enemy recognition and the costs of aggression between salamanders. Am Nat 117:962–974
Jaeger RG, Gergits WF (1979) Intra- and interspecific communication in salamanders through chemical signals on the substrate. Anim Behav 27:150–156
Jaffe K, Marcuse M (1983) Nestmate recognition and territorial behaviour in the antOdontomachus bauri Emery (Formicidae: Ponerinae). Insectes Sociaux 30:466–481
Khalil SH (1978) Development of the olfactory organ of the Egyptian toad,Bufo regularis Reuss. I. Larval period. Folia Morphol (Prague) 26:69–74
Kukuk PF, Breed MD, Sobti A, Bell WJ (1977) The contributions of kinship and conditioning to nest recognition and colony member recognition in a primitively eusocial bee,Lasioglossum zephyrum (Hymenoptera: Halictidae). Behav Ecol Sociobiol 2:319–327
Linsenmair KE (1972) Die Bedeutung familienspezifischer ‘Abzeichen’ für den Familienzusammenhalt bei der sozialen WüstenasselHemilepistus reaumuri Audouin u. Savigny (Crustacea, Isopoda, Oniscoidea). Z Tierpsychol 31:131–162
Madison DM (1975) Intraspecific odor preferences between salamanders of the same sex: dependence on season and proximity of residence. Can J Zool 53:1356–1361
Madison DM (1977) Chemical communication in amphibians and reptiles. In: Müller-Schwarze D, Mozell MM (eds) Chemical signals in vertebrates. Plenum Press, New York, pp 135–168
McGavin M (1978) Recognition of conspecific odors by the salamanderPlethodon cinereus. Copeia 1978:356–358
Nordeng H (1971) Is the local orientation of anadromous fishes determined by pheromones? Nature 233:411–413
O'Hara RK, Blaustein AR (1981) An investigation of sibling recognition inRana cascadae tadpoles. Anim Behav 29:1121–1126
O'Hara RK, Blaustein AR (1982) Kin preference behavior inBufo boreas tadpoles. Behav Ecol Sociobiol 11:43–49
Onoda N, Katsuki Y (1972) Chemoreception of the lateral-line organ of an aquatic amphibian,Xenopus laevis. Jpn J Physiol 22:87–102
Pfennig DW, Gamboa GJ, Reeve HK, Reeve JS, Ferguson ID (1983) The mechanism of nestmate discrimination in social wasps (Polistes, Hymenoptera: Vespidae). Behav Ecol Sociobiol 13:299–305
Porter RH, Wyrick M, Pankey J (1978) Sibling recognition in spiny mice (Acomys cahirinus). Behav Ecol Sociobiol 3:61–68
Quinn TP, Busack CA (1985) Chemosensory recognition of siblings in juvenile coho salmon (Oncorhynchus kisutch). Anim Behav 33:51–56
Reuter T (1969) Visual pigments and ganglion cell activity in the retinae of tadpoles and adult frogs (Rana temporaria L.). Acta Zool Fenn 122:1–64
Risser J (1914) Olfactory reactions in amphibians. J Exp Zool 16:617–652
Roberts A (1980) The function and role of two types of mechanoreceptive ‘free’ nerve endings in the head skin of amphibian embryos. J Comp Physiol 135:341–348
Roberts A, Hayes BP (1977) The anatomy and function of ‘free’ nerve endings in an amphibian skin sensory system. Proc R Soc Lond B 196:415–429
Russell IJ (1976) Amphibian lateral line receptors. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 513–550
Selset R, Døving KB (1980) Behaviour of mature anadromous char (Salmo alpinus L.) towards odorants produced by smolts of their own population. Acta Physiol Scand 108:113–122
Siegel S (1956) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Smith BH (1983) Recognition of female kin by male bees through olfactory signals. Proc Natl Acad Sci USA 80:4551–4553
Spaeti U (1978) Development of the sensory systems in the larval and metamorphosing European grass frog (Rana temporaria L.). J Hirnforsch 19:543–575
Stabell OB (1982) Detection of natural odorants by Atlantic salmon parr using positive rheotaxis olfactometry. In: Brannon EL, Salo EO (eds) Salmon and trout migratory behavior symposium. School of Fisheries, University of Washington, Seattle, pp 71–78
Stabell OB, Selset R, Sletten K (1982) A comparative chemical study on population-specific odorants from Atlantic salmon. J Chem Ecol 8:201–217
Tristram DA (1977) Intraspecific olfactory communication in the terrestrial salamanderPlethodon cinereus. Copeia 1977:597–600
Waldman B (1981) Sibling recognition in toad tadpoles: the role of experience. Z Tierpsychol 56:341–358
Waldman B (1982) Sibling association among schooling toad tadpoles: field evidence and implications. Anim Behav 30:700–713
Waldman B (1983) Kin recognition and sibling association in anuran amphibian larvae. PhD thesis, Cornell University, Ithaca, New York
Waldman B (1984) Kin recognition and sibling association among wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 14:171–180
Waldman B (1985) Sibling recognition in toad tadpoles: are kinship labels transferred among individuals? Z Tierpsychol 68:41–57
Waldman B, Adler K (1979) Toad tadpoles associate preferentially with siblings. Nature 282:611–613
Wassersug RJ (1973) Aspects of social behavior in anuran larvae. In: Vial JL (ed) Evolutionary biology of the anurans. University of Missouri Press, Columbia, pp 273–297
Wassersug R, Hessler CM (1971) Tadpole behaviour: aggregation in larvalXenopus laevis. Anim Behav 19:386–389
Wassersug RJ, Lum AM, Potel MJ (1981) An analysis of school structure for tadpoles (Anura: Amphibia). Behav Ecol Sociobiol 9:15–22
Weiss N, Hassett M (1982) Introductory statistics. Addison-Wesley, Reading, Massachusetts
Wills GD, Wesley AL, Sisemore DA, Anderson HN, Banks LM (1983) Discrimination by olfactory cues in albino rats reflecting familiarity and relatedness among conspecifics. Behav Neural Biol 38:139–143
Wilson EO (1968) Chemical systems. In: Sebeok TA (ed) Animal communication. Indiana University Press, Bloomington, pp 75–102
Witschi E (1949) The larval ear of the frog and its transformation during metamorphosis. Z Naturforsch 4B:230–242
Wu HMH, Holmes WG, Medina SR, Sackett GP (1980) Kin preference in infantMacaca nemestrina. Nature 285:225–227
Wysocki CJ (1982) The vomeronasal organ: its influence upon reproductive behavior and underlying endocrine systems. In: Breipohl W (ed) Olfaction and endocrine regulation. IRL Press, London, pp 195–208
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Waldman, B. Olfactory basis of kin recognition in toad tadpoles. J. Comp. Physiol. 156, 565–577 (1985). https://doi.org/10.1007/BF00619107
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00619107