Abstract
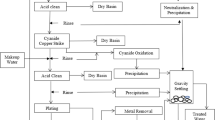
The effectiveness of a new process for recovering copper ions from dilute solutions has been tested. Porous, fixed, flow-through graphite electrodes are used in conjunction with a membrane which separates the two feed streams. The concept is demonstrated using a dilute feed of 800μ ml{−1} copper ions which is reduced to less than 1μ ml{−1}, and a concentrated stream of 0.4 M copper which is concentrated to 0.7M. The results show the concept is technically feasible and that it is competitive with existing technology for copper recovery.
Similar content being viewed by others
Abbreviations
- a :
-
area per unit volume, cm−1
- c o :
-
copper concentration of feed, mol cm−3
- c b :
-
averaged, bulk copper concentration within porous cathode, mol cm−3
- c Lb :
-
copper concentration in cathode effluent, mol cm−3
- E 0 :
-
potential difference between reversible hydrogen reference electrode and reversible copper reference electrode in solution aty equal zero, V.
- F :
-
Faraday's constant, 96487 coul/equiv
- i 2 :
-
superficial or overall electrical current density in catholyte solution, A cm−2
- i t :
-
total, overall current density to cathode, A cm−2
- j :
-
transfer current density, A cm−2
- k m :
-
mass transfer coefficient, cm s−1
- L :
-
thickness of the cathode, cm
- M:
-
molecular weight, g gmol−1
- n :
-
number of electrons transferred in electrode reaction, 2
- q e :
-
charge passed per unit volume of electrode, coul cm−3
- Re :
-
Reynolds number, dvρμ −1
- Sc :
-
Schmidt numberv D−1, dimensionless
- t :
-
time to plug cathode with copper,s
- v :
-
superficial or approach velocity of catholyte solution, cm s−1
- VDP:
-
potential of saturated calomel reference electrode in catholyte effluent relative to the cathode, V
- VA:
-
potential of anode relative to cathode, V
- y :
-
distance from entrance of cathode toward cathode backing plate, cm
- α :
-
ak m/v, cm−1
- β :
-
nFv 2 c o /ak m K, V
- ε :
-
void fraction, dimensionless
- k :
-
effective or superficial electrical conductivity of catholyte, mho cm−1
- μ :
-
viscosity of feed solution, g cm−1 s−1
- v :
-
kinematic viscosity, cm2 s−1
- ρ :
-
density, g cm−3
- φ 1 :
-
potential in the matrix, V
- φ 2, b :
-
potential plus a constant of a reversible hydrogen electrode in the solution, V
- φ 2s :
-
potential of a reversible copper electrode adjacent to pore wall, V
- δφ ohmic :
-
potential difference in bulk solution due to current flow, V
- ψ :
-
particle shape factor, 0.86 for flakes, dimensionless
- o:
-
front of the electrode
- 1:
-
solid phase
- 2:
-
solution phase
- b :
-
bulk solution
- s :
-
solid-solution interface
References
Deco Trefoil, ‘Copper-hydrometallurgy’, vol. 33, no. 4, Bulletin No. G3-B146 (1969) 9.
D. W. Agers, J. E. House, R. R. Swanson and J. L. Drobnick, ‘Copper Recovery from Acid Solutions using Liquid Ion Exchange’, General Mills Chemicals, Inc., Minneapolis, Company Report, (not dated).
D. W. Agers, and E. R. DeMent, ‘LIXR64 As an Extractant for Copper’, Presented at Fall Meeting of the Society for Mining Engineers of AIME, Las Vegas, Nevada, Sept 6–8, 1967, abstract inMm. Eng. 19 (1967) 25.
G. A. Carlson, U.S. 3, 459, 646; 5 Aug. 1969.
Idem, U.S. 3,647,653; 7 March 1972.
Idem, U.S. 3,650,925; 21 March 1972.
F. A. Posey, ORNL-IM-4112, March, 1973.
D. N. Bennion and J. Newman,J. Appl. Electrochem. 2 (1972) 113.
R. Alkire and P. K. Ng,J. Electrochem. Soc. 121 (1974) 95.
A. K. P. Chu, M. Fleischman and G. J. Hills,J. Appl. Electrochem. 4 (1974) 323.
A. K. P. Chu and G. J. Hills,ibid 4 (1974) 331.
Gracon, ‘Flow Through Porous Electrodes’, PhD dissertation, University of Illinois, Urbana (1975).
G. B. Adams, R. P. Hollandsworth and D. N. Bennion,J. Electrochem. Soc. 122 (1975) 1043.
R. S. Wenger, ‘Electrochemical Concentrating and Purifying from Dilute Copper Solutions’, M.S. Thesis, University of California, Los Angeles, Feb. 1974.
R. B. Bird, W. E. Steward and E. N. Lightfoot, ‘Transport Phenomena’, John Wiley & Sons, New York (1960) pp. 411 and 679.
A. J. Karabelas, T. H. Wegner and T. J. Hanratty,Chem. Eng. Sci.,26 (1971) 1581.
J. A. Mandelbaum, and U. Böhm,ibid,28 (1973) 569.
J. E. Williamson, K. E. Bazaire and C. J. GeankoplisInd. Eng. Chem.,2 (1963) 126.
E. I. Wilson, and C. J. Geankoplis,ibid 5 (1966) 9.
L. K. McCune, and R. H. Wilhelm,ibid 41 (1949) 1124.
M.S. Peters, and Klaus D. Timmerhaus, ‘Plant Design and Economics for Chemical Engineers’, 2nd edn., McGraw-Hill (1968) pp. 104 and 131.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wenger, R.S., Bennion, D.N. Electrochemical concentrating and purifying from dilute copper solutions. J Appl Electrochem 6, 385–396 (1976). https://doi.org/10.1007/BF00616537
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00616537