Abstract
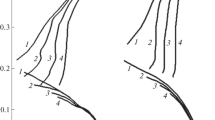
Graphite electrodes were prepared by mixing calcined coke and coal tar pitch. They were pressed under 250 kg cm−2 and heat treated up to 2800° C. Rectangles measuring 70 mm x 40 mm x 8 mm were anodically polarized under galvanostatic and potentiostatic conditions. Electrolyses were conducted at 10–50 mA cm−2 for periods ranging from 10–120 hours in Na2SO4 solutions acidified with sulphuric acid to various pH values. The wear of graphite anodes increased with decreasing bath temperature, increasing acid concentration, decreasing pH of the electrolyte and increasing current density. A model is suggested which assumes that corrosion takes place via the formation of a lamellar crystal compound with the formula (C 08 O)(OH)3HSO −4 ·2H2SO4.
The compound is unstable at higher temperatures when corrosion is effected by oxidation of graphite by atomic oxygen. The formation of the carbon ions was found to be a necessary precondition for the formation of the complex.
Similar content being viewed by others
References
L. I. Krishtalik,Elektrokhim. 2 (1966) 393.
V. I. Eberil, D. V. Kokoulina, L. I. Krishtalik and L. M. Elina,ibid 5 (1969) 336.
A. Korczynski and R. Dylewski,Chemik 22 (1969) 421.
F. Rokuro,Denkt Kagaku 40 (1972) 380.
Vignaud,J. Chim. Phys. Physiochim. Biol. 67 (1970) 973.
M. C. Robert, M. Oberlin and J. Mering, ‘Chemistry and Physics of Carbon’, Vol. 10, Marcel Dekker, New York (1973) p. 155.
R. Fujii,Nippon Kagaku Kaishi 11 (1975) 1888.
N. D. Viet, D. V. Kokoulina and L. I. Krishtalik,Elektrokhim. 8 (1972) 387.
A. Korczynski and R. Dylewski,Zesz. Nauk Politech. Salsk. Chem. 29 (1966) 67.
Idem, Chemik 22 (1969) 421.
G. N. Kokhanov and R. A. Agapova,Zh. Prikl Khim. 46 (1973) 1231.
G. N. Kokhanov and L. A. Khanova,Elektrokhim. 8 (1972) 1159.
M. A. Rabah, A. A. Abdul Azim and S. Z. El-Tawil, to be published.
G. Wranglén, B. Sjodin and B. Wallen,Electrochim. Acta 7 (1962) 577.
P. A. V. Wiggs, ‘Industrial Carbon and Graphite Papers’, Chemical Society, London (1958) p. 253.
P. L. Walker, Jr., F. Rusinko and E. Raats,J. Phys. Chem. 59 (1955) 245.
M. M. Dubinin, ‘Industrial Carbon and Graphite Papers’, Chemical Society, London (1958) p. 219.
X. Ritter and X. Drake,Ind. Eng. Chem. Anal. Ed. 17 (1945) 782.
G. N. Kokhanov and L. A. Khanova,Elektrokhim. 6 (1970) 866.
H. P. Boehm, M. Eckel and W. Scholz,Z. Anorg. Allg. Chem. 353 (1967) 236.
D. V. Kokoulina and L. I. Krishtalik,Elektrokhim. 3 (1967) 848.
A. R. Ubbelohde and F. A. Lewis, ‘Graphite and its Crystal Compounds’, Oxford University Press, London (1960) 110.
‘Industrial Carbon and Graphite Papers’, Chemical Society, London (1958) p. 302.
H. Thiele,Z. Anorg. Chem. 190 (1930) 145.
R. E. Franklin,J. Chim. Phys. 50 (1953) C26.
U. Hofmann,Ergebn. Exaxt. Naturz. 18 (1939) 229.
‘Industrial Carbon and Graphite Papers, Chemical Society, London (1958) p. 308.
A. A. Ravazyan,Tsvet. Metal. 43 (1970) 50.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rabah, M.A., Abdul Azim, A.A. & Ismail, A. Wear of graphite anodes during electrolysis of add sulphate solutions. J Appl Electrochem 11, 41–47 (1981). https://doi.org/10.1007/BF00615320
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00615320